Sequestration of tissue-resident alloreactive T cells after intrahepatic activation contributes to tolerance induction
Moumita Paul-Heng1, Eric T Son1, Shivanjali Ratnaseelan1, Martina Denkova1, Chuanmin Wang1, Tom Ashhurst2, Anthony W Purcell3, Nicole L La Gruta3, Nicole A Mifsud3, Alexandra Sharland1.
1Central Clinical School, The University of Sydney, Sydney, Australia; 2School of Medical Sciences, The University of Sydney, Sydney, Australia; 3Biochemistry and Molecular Biology, Monash University, Melbourne, Australia
Introduction: Our understanding of T cell-mediated alloresponses has been limited by the lack of information about allogeneic pMHC epitopes. Recently, our group identified over 40 H-2Kb-peptide epitopes that are directly recognised by CD8+ T cells from allogeneic B10.BR (H-2k) or Balb/c (H-2d) mice. Here, we aimed to characterise the TCR repertoire responding to these epitopes during skin graft rejection or tolerance induction.
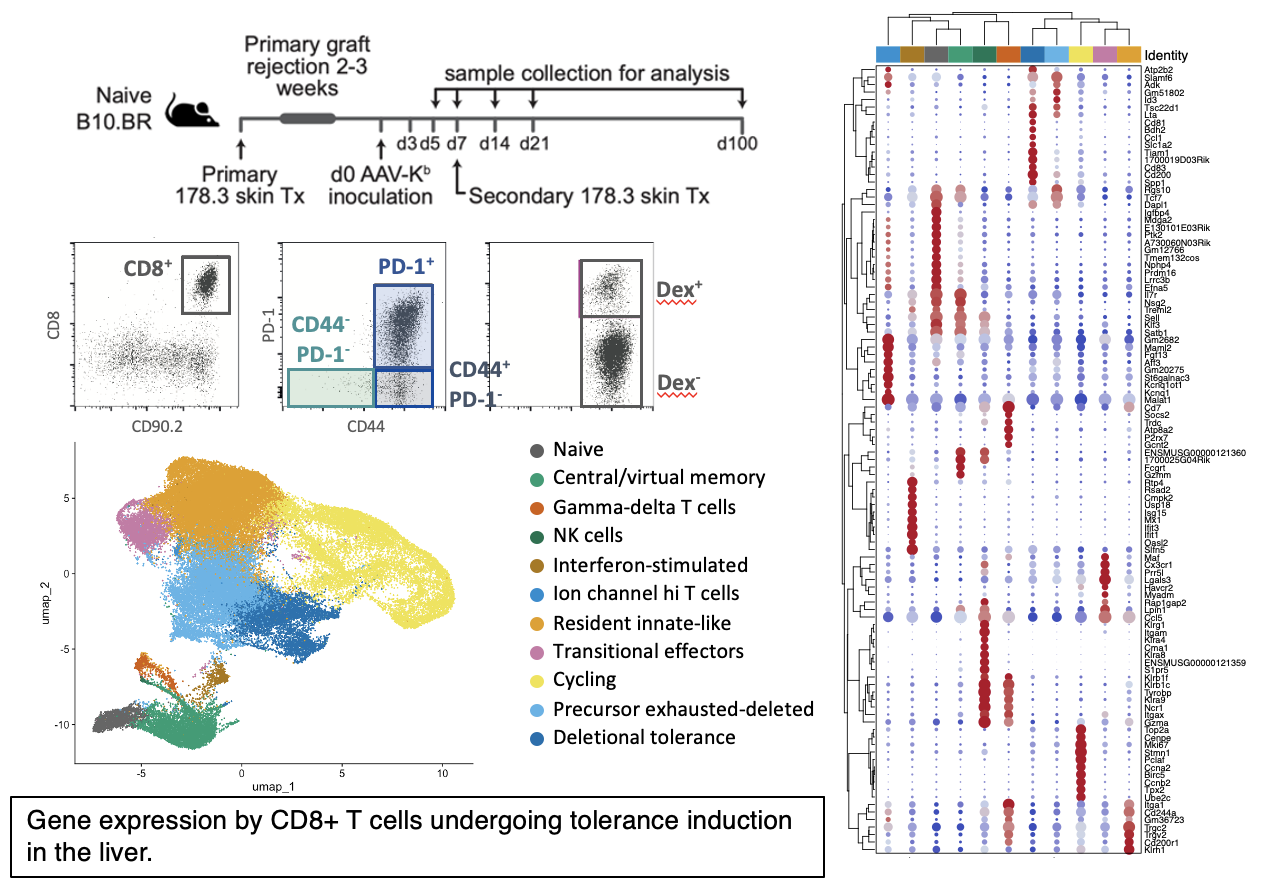
Methods: B10.BR mice underwent secondary skin grafts from transgenic donors mismatched for H-2Kb, with or without prior treatment with a liver-specific AAV vector encoding Kb heavy chain. AAV-Kb treatment induced indefinite graft acceptance. Activated CD8+ T cells were isolated from the liver at intervals from d3-d100 post-inoculation, while infiltrating lymphocytes were isolated from rejecting or accepted skin grafts on d14. Single cells were captured and TCR/transcriptome/surface marker libraries prepared. Specificity was assessed using a panel of 12 Kb-peptide dextramers.
Results: Within the liver CD8+ T cell compartment, cycling cells and cells in the deletional tolerance clusters became progressively less frequent with increasing time post AAV-Kb inoculation, while the proportion of tissue resident cells expressing an “exhausted” gene signature increased. By d14, tissue-resident cells outnumber other populations. Previously-identified public clonotypes were present early during tolerance induction, but declined as a proportion of liver alloreactive T cells as the duration of tolerance increased, suggesting that these clones may be preferentially deleted. CD4+ Tregs were over-represented in accepted skin grafts while the infiltrate of rejecting grafts featured cytotoxic effectors (NK and CD8+ T cells).
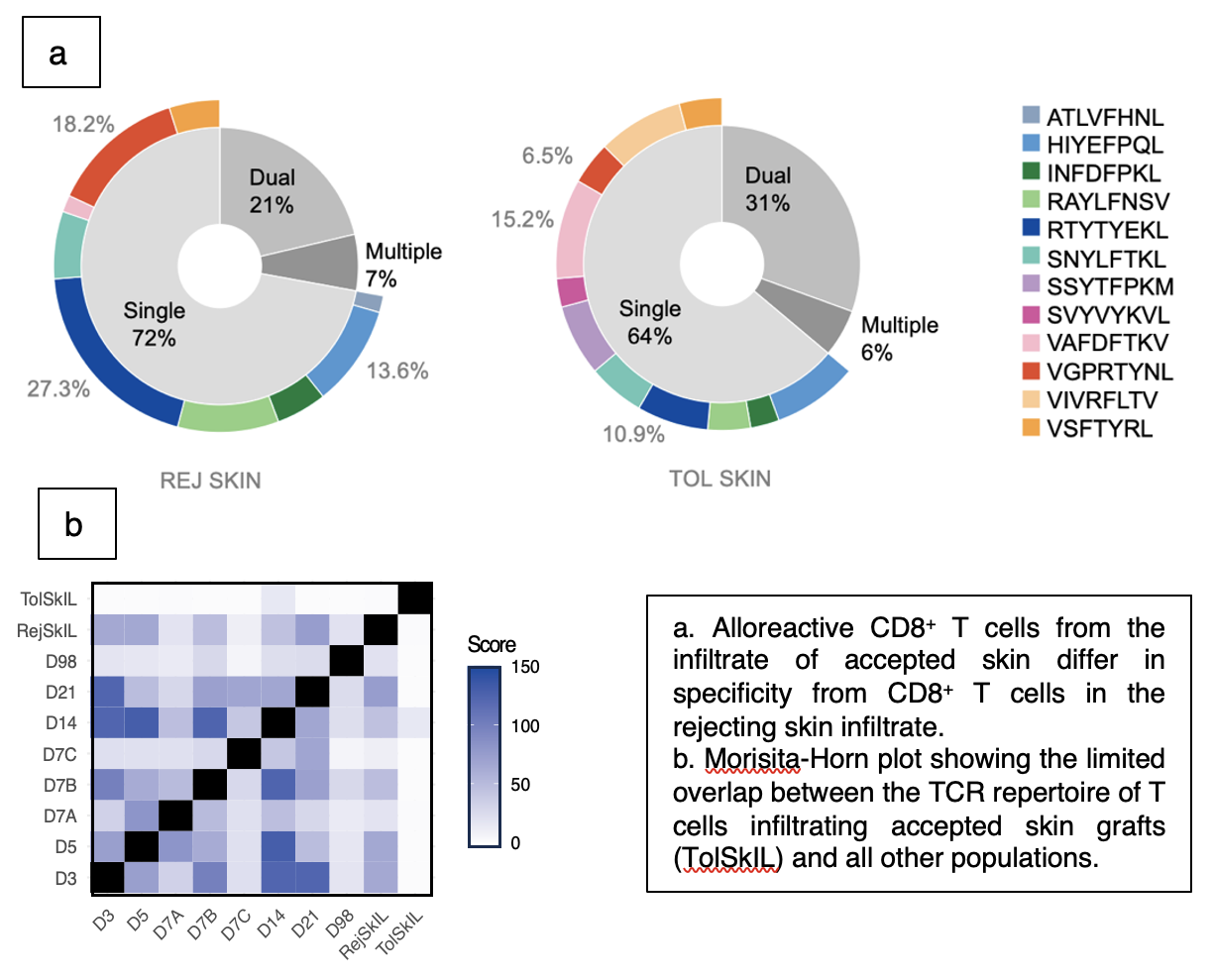
Rejecting skin infiltrate is characterized by presence of tetramer-bright (high avidity) cells, and by a high proportion of clonotypes recognizing H-2Kb in complex with RTYTYEKL, VGPRYTNL or INFDFPKL. In accepted skin grafts, cells specific for these epitopes are supplanted by cells recognizing Kb-VAFDFTKV, Kb-VIVRFLTV or Kb-SSYTFPKM. To determine the overlap between the skin and liver repertoires, we calculated the Morisita-Horn index for all samples. Minimal overlap was noted between the T cell infiltrate in accepted skin and any other repertoires. Abundant public clonotypes were absent from accepted skin, even when present among the liver T cell population from the same individuals, suggesting that sequestration of alloreactive T cells with a tissue-resident phenotype within the liver may contribute to acceptance of remote tissues.
Conclusions: Multiple mechanisms contribute to the induction of tolerance to allogeneic skin grafts following expression of donor MHC I in recipient hepatocytes. In addition to deletion and exhaustion, prevention of recirculation by liver-resident T cells may impede skin graft rejection. CD4+ regulatory T cells may also contribute to mediating skin graft acceptance.
National Health and Medical Research Council of Australia, Ideas Grant 1183806. Myee Codrington Medical Research Foundation. Royal Prince Alfred Hospital Transplant Institute.
[1] MHC class I
[2] allorecognition
[3] graft rejection
[4] tolerance
[5] T cell
[6] transcriptome
[7] immunopeptidome
[8] TCR repertoire
[9] pMHC specificity
[10] single cell multiomics
