Nora Salah Atallah, United Kingdom has been granted the TTS-FOCIS Basic and Translational Mentee-Mentor Award
Molecular and spatial profiling of GMP-Grade Tregs in kidney transplantation
Nora Atallah1, Oliver McCallion1, Sandeep Kumar3, Matthew Brook1,2, Paul Harden2, Joanna Hester1, Fadi Issa1.
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom; 2Oxford Transplant Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom; 3Guy's and St Thomas' Hospital, NHS Foundation Trust, London, United Kingdom
The TWO Study.
Background: Regulatory T cell (Treg) therapy holds promise for enhancing outcomes after solid organ transplantation. In the TWO Study, a Phase II clinical trial, we investigate ex vivo expanded polyclonal Treg therapy in living donor kidney transplantation. Alongside characterizing the homing potential and molecular phenotype of GMP-grade Tregs, we employ high-plex Xenium in situ analysis to map spatial expression signatures in different tissue regions of Treg cell therapy patients. This advanced spatial profiling technique allows for a detailed examination of the interactions between Tregs and the surrounding tissue environment at a single cell level.
Methods: Tregs were isolated and expanded according to a standardised protocol at Guy’s and St. Thomas’s Hospital GMP Unit. Chemokine expression and secretion were detected via flow cytometry, and Nanostring nCounter was used for transcriptomic analysis. Additionally, high-plex Xenium in situ analysis was utilised for high resolution spatial expression signatures profiling in different tissue regions of Treg treated patients versus control patients who did not receive Treg therapy.
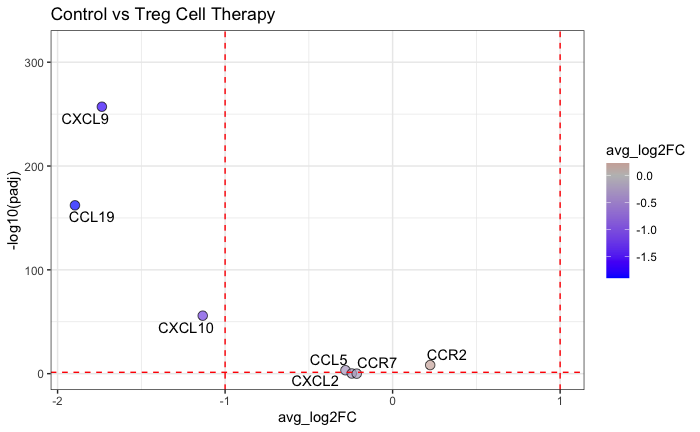
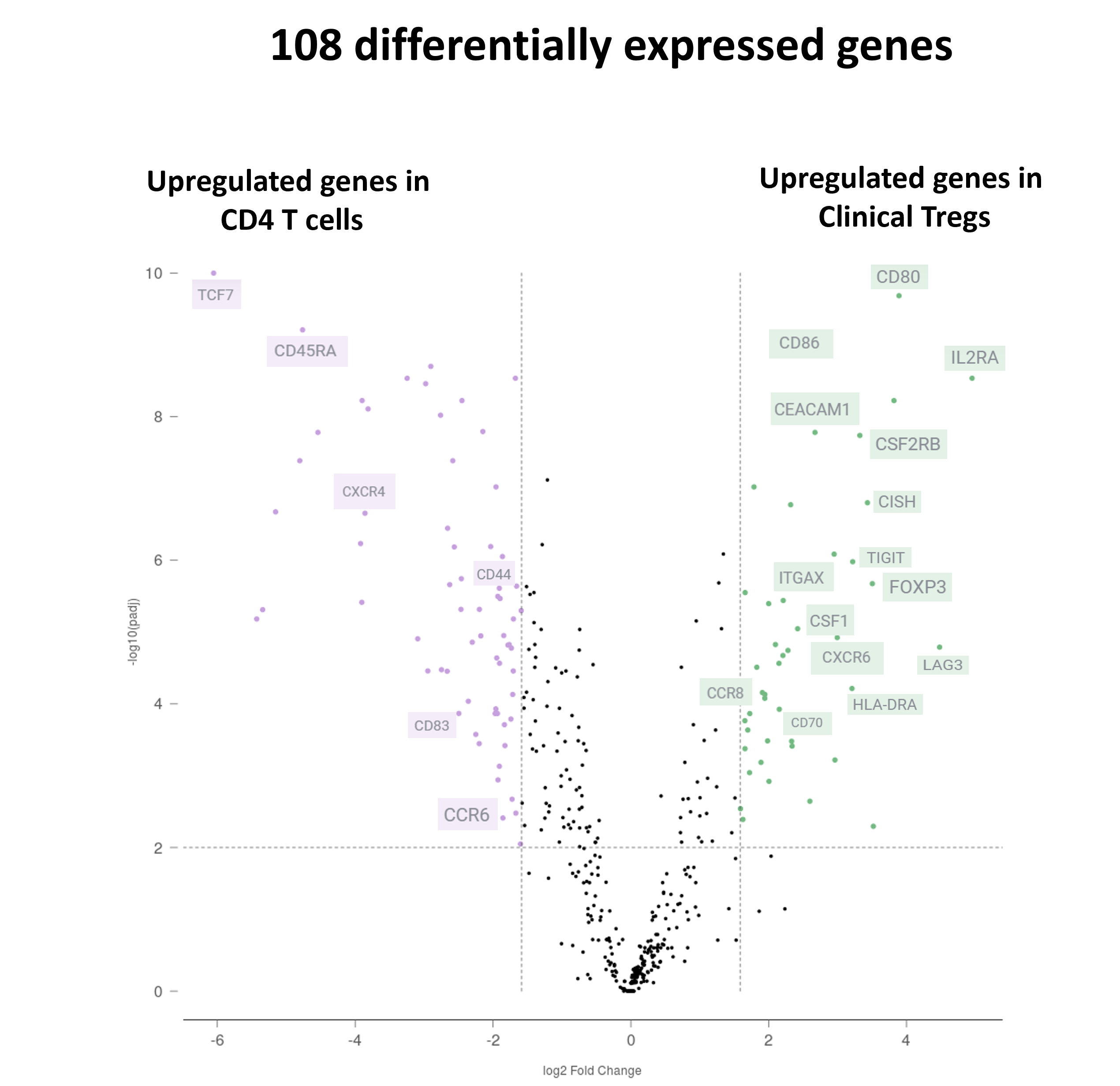
Results: Clinical-grade Tregs exhibited typical Treg marker expression and upregulated cell adhesion/migration molecules, including increased expression of CXCR6 and CCR8 and a lower expression of CXCR4 compared with conventional T cells. Spatial analysis demonstrated that the administration of Tregs reduces the tissue expression of key chemokine ligand genes in areas of Treg infiltration, in particular CXCL9, CXCL10, and CCL19.
Conclusions: Clinically expanded Tregs retain Treg-typical molecular characteristics and express homing receptors, potentially supporting their trafficking to inflamed areas. Incorporating spatial expression signatures provides additional insights into the unique microenvironments and multiplexed protein expression at subcellular resolution in the context of Treg therapy for solid organ transplantation. The downregulation of CXCL9, CXCL10, and CCL19 following Treg infusion suggests a potential reduction of the overall inflammatory response, although specific mechanisms require clarification. Further analysis is ongoing to examine these specific Treg interactions within the tissue.
MR/N027930/1/MRC_/Medical Research Council/United Kingdom .
[1] Tregs, cell therapy, gene expression, chemokine receptors, solid organ transplantation, Xenium
