Liver transplantation with delayed donor bone marrow transplantation in non-human primates achieves hematopoietic chimerism
Katsuhiro Tomofuji1, Olivia Bourgeois1, Ahmad Karadagi1, Ryo Otsuka1, Toshihide Tomosugi1, Abbas Dehnadi1, Rudy Matheson1, Anil Kharga1, James Markmann2, Tatsuo Kawai1, Shoko Kimura1.
1Transplantation / Surgery, Massachusetts General Hospital, Boston, MA, United States; 2Transplant Surgery, University of Pennsylvania, Philadelphia, PA, United States
Introduction: The ultimate goal of organ transplantation is the induction of specific immunologic tolerance. Although hematopoietic chimerism induction has successfully induced tolerance in clinical kidney transplantation, its application to liver transplant has not been previously demonstrated. We have recently developed a novel bone marrow transplant (BMT) conditioning regimen by using peri-transplant Bcl-2 inhibitor (Bcl-2i) in nonhuman primates (NHPs). Since Bcl-2i selectively deletes hematopoietic stem cells in the bone marrow niches without deleting peripheral myeloid cells, mixed chimerism is inducible without severe myelosuppression. By applying this Bcl-2i-based conditioning regimen, we present evidence of long-term immunosuppression-free survival of liver allografts following the induction of mixed chimerism in NHPs.
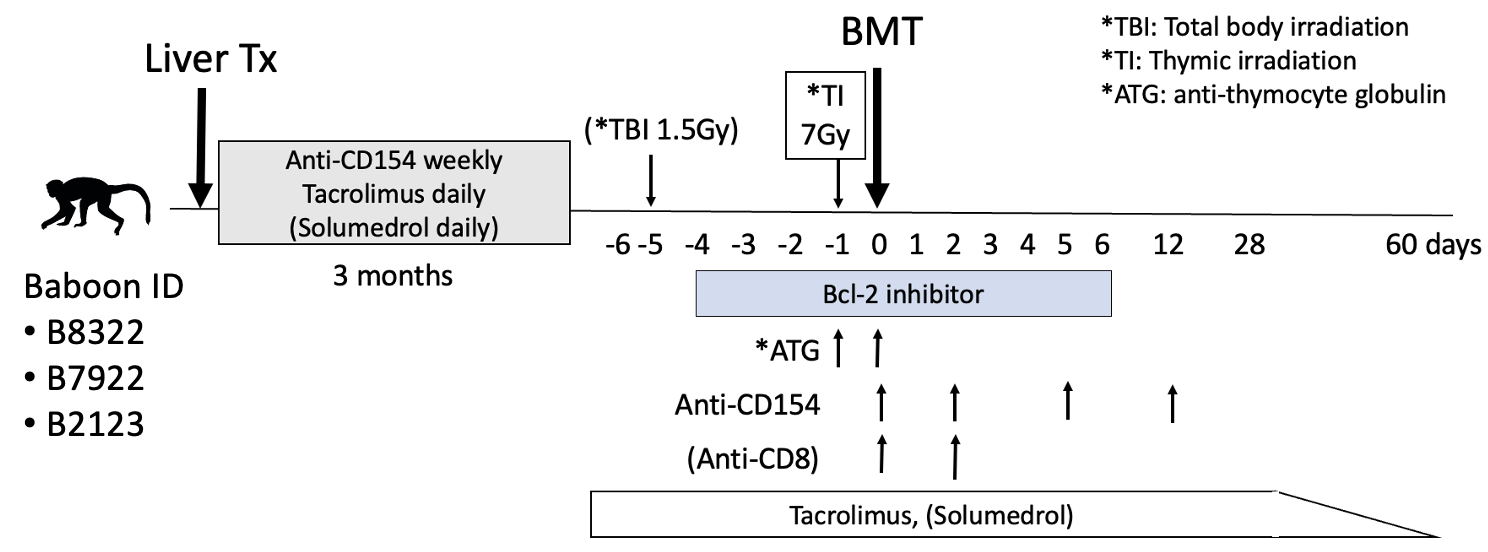
Method: Three baboons received allogenic orthotopic liver transplantation and were treated with an immunosuppressive regimen (anti-CD154 antibody, tacrolimus, and steroid). The recipients received a delayed BMT with conditioning immunosuppression therapy 3 months after liver transplantations. All three recipients were treated with Bcl-2i based BMT conditioning regimens which included peri-transplant Bcl-2i, anti-thymocyte globulin (ATG), anti-CD154 antibody, thymic irradiation, and a two-month post-BMT course of tacrolimus. All immunosuppression medications were discontinued 2 months after BMT. As the first recipient failed to develop mixed chimerism, low dose (1.5Gy) total body irradiation (TBI) and anti-CD8 antibody were added in the second recipient. The third recipient received TBI without anti-CD8 antibody.
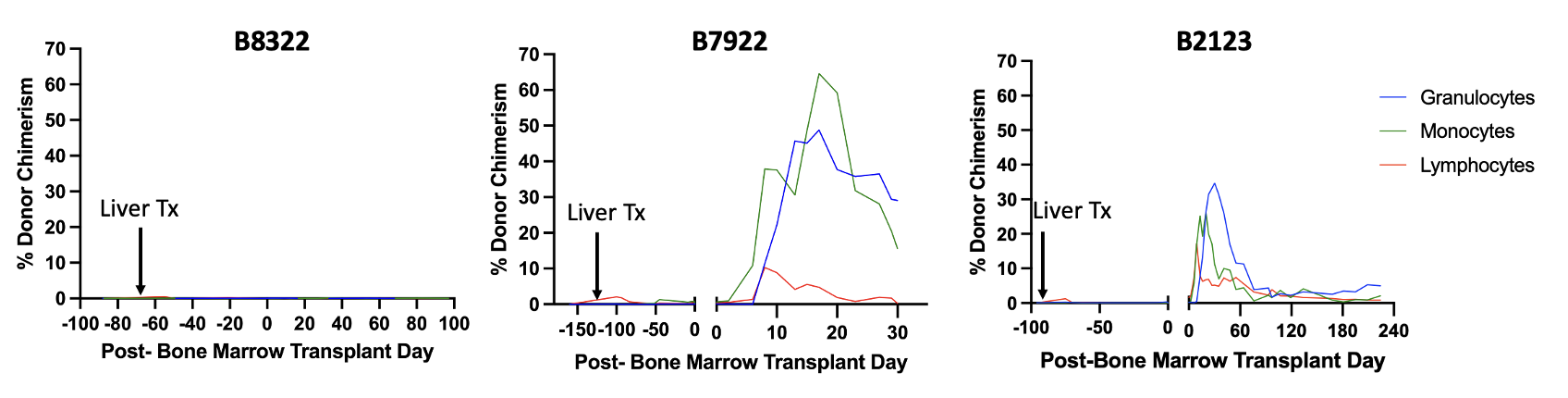
Results: Immediate post-operative courses of all three liver transplants were uneventful. The first recipient (B8322) failed to develop mixed chimerism and rejected the liver while tapering off immunosuppression (57 days after BMT). By adding low-dose TBI and anti-CD8 antibody, the second recipient (B7922) successfully developed mixed chimerism (Lymphocytes 10.3%, Granulocytes 48.8%, Monocytes 64.6%). However, the recipient was lost due to sepsis secondary to cholangitis 30 days after BMT. Therefore, in the third recipient (B2123), anti-CD8 antibody was omitted. This recipient successfully developed mixed chimerism (Lymphocytes 17.2%, Granulocytes 34.7%, Monocytes 26.3%) and achieved long-term immunosuppression-free liver allograft survival (>224 days after BMT). No donor specific antibody has been detectable and a liver biopsy on post-op day 216 only showed non-specific mild portal tract inflammation.
Conclusion: Induction of mixed chimerism and long-term immunosuppression-free liver allograft survival have been achieved via delayed BMT using a Bcl-2i based conditioning regimen.
This work is presented at American Transplant Congress 2024 (June-4-2024).
[1] non-Human Primates
[2] Liver
[3] Chimerism
[4] Tolerance
[5] Bone Marrow Transplant
