Successful mixed hematopoietic chimerism by Bcl-2 Inhibition and rapamycin encapsulating nanoparticles (ImmTOR) without chemotherapy or total body irradiation in nonhuman primates
Ahmad Karadagi1, Toshihide Tomosugi1, Ryo Otsuka1, Grace Lassiter1, A Benedict Cosimi1, Kei Kishimoto2, Tatsuo Kawai1.
1Center for Transplantation Sciences, Massachusetts General hospital and Harvard Medical School, Boston, MA, United States; 2Selecta Biosciences, Inc., Watertown, MA, United States
Introduction: We have previously shown that transient expansion of donor hematopoietic cells through donor bone marrow transplantation can lead to renal allograft tolerance in non-human primates (NHP). Recently, we discovered that Venetoclax, a selective Bcl-2 inhibitor, significantly promote chimerism induction and renal allograft tolerance with reduced requirement of TBI. To completely eliminate the requirement of TBI or chemotherapy, we hypothesized that if allogeneic hematopoietic stem cells could be better protected from innate and adaptive inflammatory responses, allogeneic stem cells would more effectively engraft, even in the limited space created by Venetoclax. To explore this possibility, we evaluated ImmTOR, synthetic biodegradable nanoparticles encapsulating rapamycin in the current study.
Methods: Eight cynomolgus Macaques received combined kidney and bone marrow (2-3.8 x108 cells/Kg) transplants from MHC-mismatched donors. Six recipients received ImmTOR nanoparticles (1-3mg/kg) and venetoclax (10mg/kg for total of 11 doses). Recipients were also conditioned with anti-thymocyte globulin, 7Gy local thymic irradiation and anti-CD154 mAb, all of which have been shown essential to induce allograft tolerance in our previous studies. ImmTOR was not administered in two recipients as a control. All immunosuppression was discontinued post-operative day 28. Peripheral blood mononuclear cells from nonhuman primates were used for in vitro comparison of ImmTOR nanoparticles and free rapamycin.
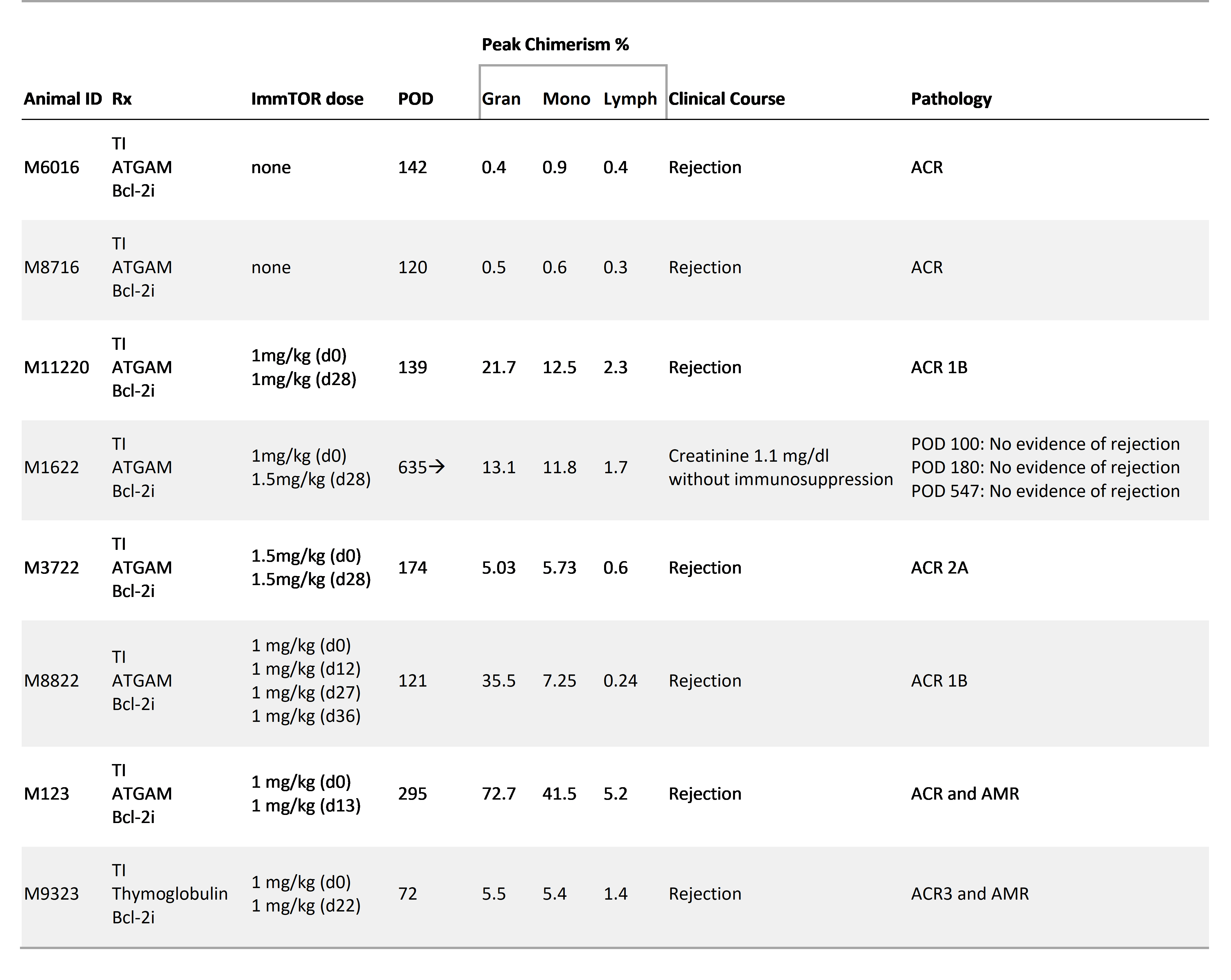
Results: Without ImmTOR, clear chimerism was not detected and both recipients rejected kidney allograft early. Mixed chimerism was consistently induced in all recipients with ImmTOR. (Table 1). The recipients tolerated the protocol well with minimal myelosuppression (fig. 1A) and without major infectious complications. Long-term immunosuppression-free renal allograft survival was achieved in two recipients (295 and 635 days). Nevertheless, four other recipients failed to achieve long-term allograft survival despite successful chimerism induction. In vitro studies, to elucidate the mechanisms of promotion of chimerism without TBI, revealed ImmTOR treated CD14+ monocytes with increased PD-L1 expression and decreased HLA-DR by flow cytometry. Conversely, this effect was not observer in cells treated with free rapamycin, possibly indicating the preferential uptake of antigen presenting cells and modified mechanism of action of nanoparticle encapsulated rapamycin (fig. 1B).
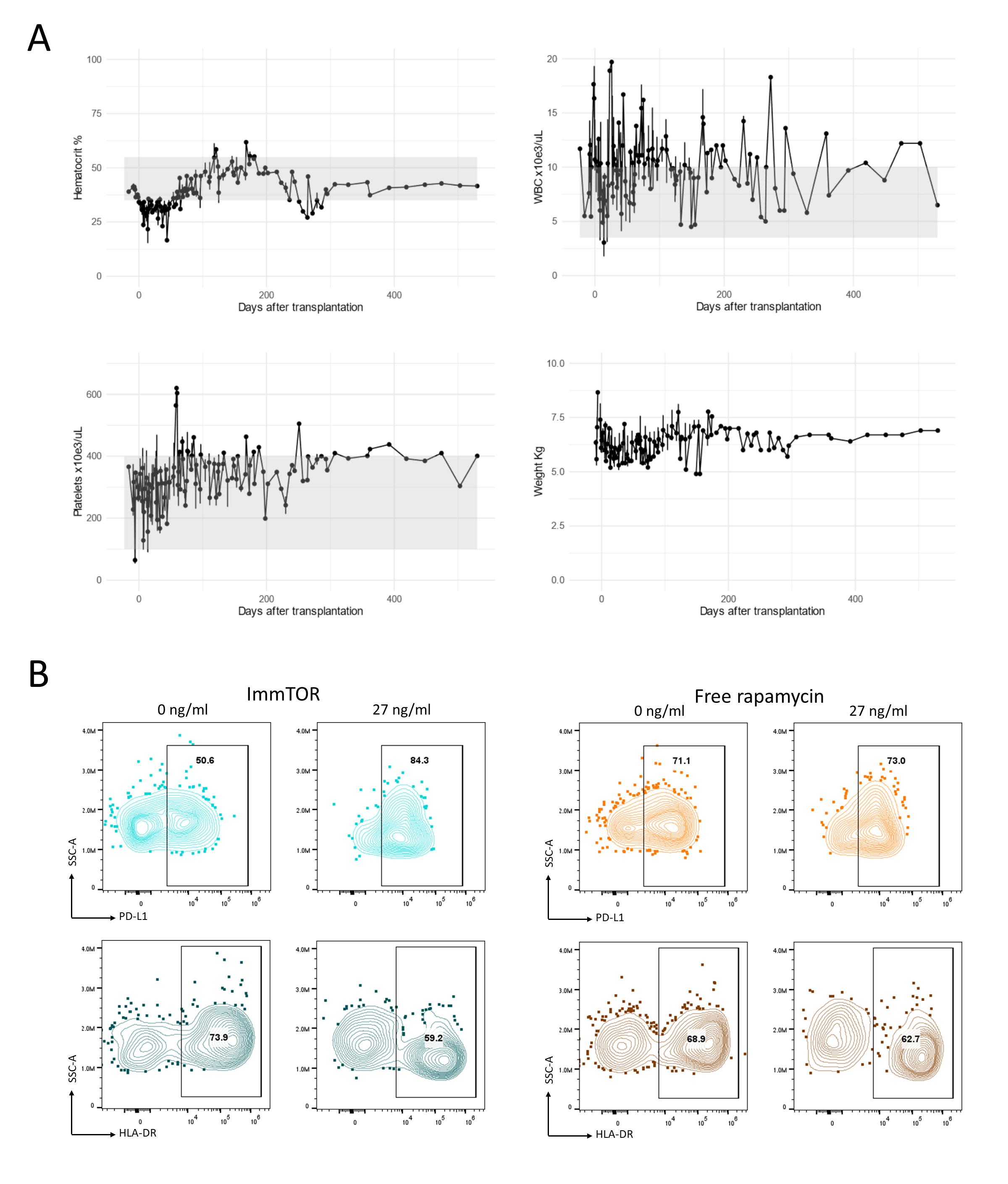
Conclusions: Hematopoietic chimerism was induced by the combination of Bcl-2i and ImmTOR nanoparticles, for the first time without TBI or chemotherapy. The nanoparticles may target the antigen presenting cell preferentially and exercises the immunosuppression selectively. Immunological tolerance appears to have been achieved in one animal, but further optimization of this protocol is needed for consistent tolerance induction. Nonetheless, Bcl-2i/ImmTOR is promising approach to induce allograft tolerance via mixed chimerism.
