Generation of CD141+CD163+ regulatory dendritic cells from live donor liver transplant perfusate
Rosalia Busà1,2, Alan F Zahorchak2, Camila Macedo2, Diana M Metes2, Ester Badami3, Beth Elinoff2, Fadi G Lakkis2, Abhinav Humar2, Pier Giulio Conaldi1, Angus W Thomson2.
1Research Department, IRCCS-ISMETT, Palermo, Italy; 2Starzl Transplantation Institute, University of Pittsburgh, Pittsburgh, PA, United States; 3Research Department, Fondazione Ri.MED, Palermo, Italy
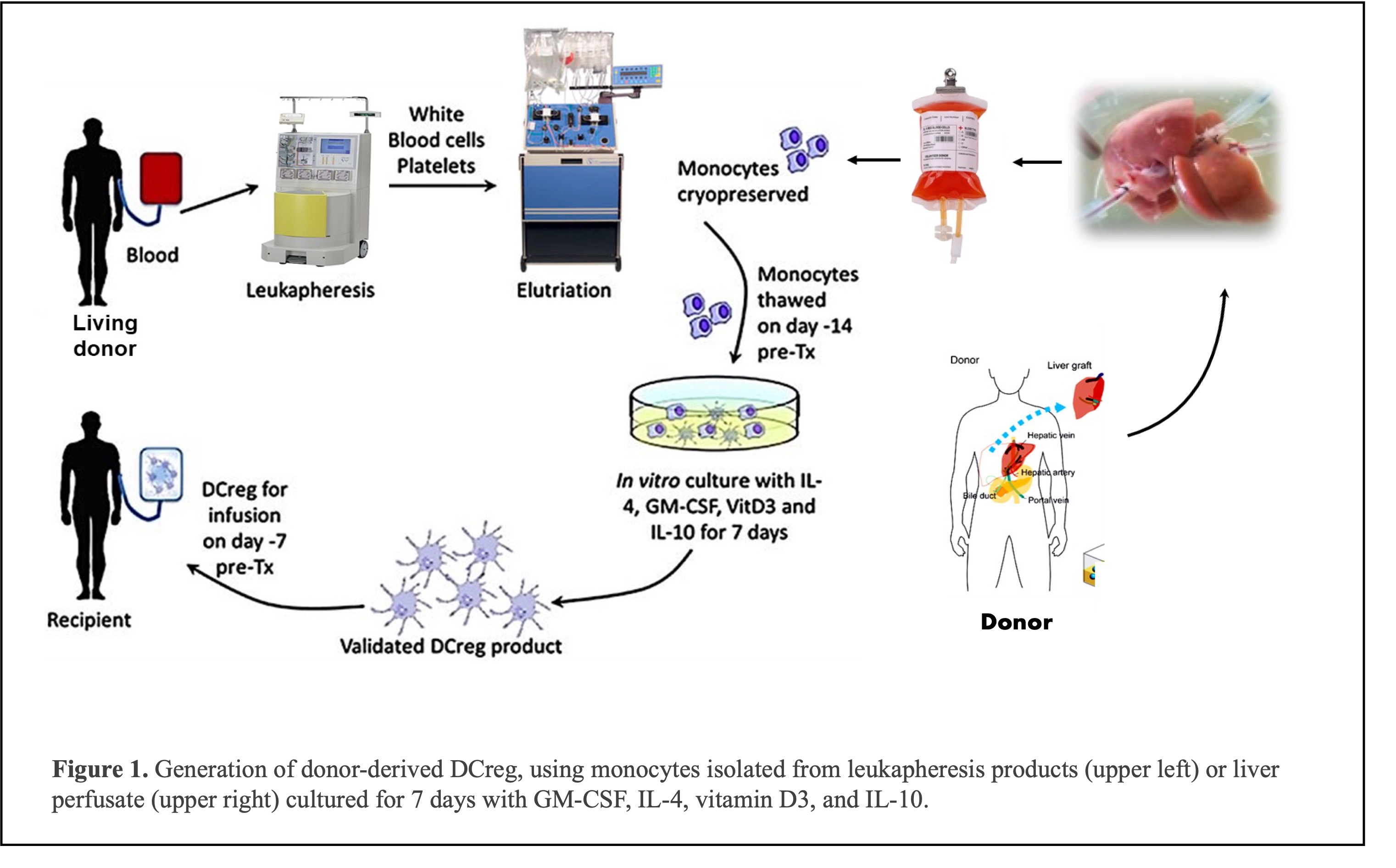
Introduction: Regulatory dendritic cells (DCregs) have emerged as promising cellular therapeutics for reducing host dependence on immunosuppressive drugs and enhancing organ transplant tolerance. The feasibility, safety, and immune modulatory activity of DCregs generated ex vivo from donor blood monocytes isolated from leukapheresis products have been validated in early trials of live donor liver transplantation. To potentially expand the application of adoptive DCreg therapy to deceased donor liver transplantation, we explored the use of allograft perfusate as an alternative source of DCreg precursors. This study aimed to establish a protocol for DCreg generation starting from CD14+ monocytes isolated by live donor liver perfusate (LDLP) and to assess the phenotype and immunoregulatory properties of these liver perfusate-derived DCregs (LP-DCregs).
Methods: DCregs were generated from CD14 immunobead-isolated LDLP monocytes from 8 healthy adult donors in the presence of GM-CSF, IL-4, vitamin D3, and IL-10 for 7 days. Flow cytometric analysis (cell surface and intracellular staining) was used to characterize the LP-DCregs and to compare them with control DC populations. Expression of T cell co-stimulatory and co-inhibitory molecules was determined by flow cytometry and cytokine production using Luminex technology. The LP-DCregs' ability to attenuate allogeneic T cell proliferation and regulatory T cell (Treg) generation in MLR was assessed.
Results: LP-DCregs showed >80% viability, >90% purity (lin- HLA-DR+ CD11c+) with <1% T or B lymphocyte contamination. Recovery rates were higher for LP-DCreg (28-31%) than DCreg generated from blood monocytes (20-23%). LP-DCreg (CD141+CD163+) were phenotypically immature and resisted maturation when exposed to a potent pro-inflammatory stimulus (the Toll-like receptor 4 ligand monophosphoryl lipid A; MPLA), maintaining expression of HLA-DR, CD11b, CD11c, CD40, CD86 and programmed death ligand-1 (PD-L1) >90%. However, co-inhibitory:co-stimulatory molecule MFI expression ratios, PD-L1:CD86, PD-L1:CD80, and PD-L1:CD40, were all elevated significantly on LP-DCreg compared to immature DC. Quantification of multiple cytokines in 7-day cultures revealed comparable cytokine levels in DCreg culture supernatants before and after MPLA stimulation. In particular, we found minimal levels of TNF⍺ and IL12p40 and high levels of IL-10 in DCreg and DCreg+MPLA compared to immature DC and mature DC cultures resulting in high IL-10:TNF⍺ and high IL-10:IL12p40 ratios. Functional assessment performed in MLR assay revealed that LP-DCregs induced T cell unresponsiveness by suppression of effector CD4 and CD8 T cell proliferation and induction of Tregs (CD4+CD25+Foxp3+CD127-), even after TLR4 ligation with MPLA.
Conclusions: These novel preliminary observations reveal that human LDLP monocytes are promising sources of DCreg for potential evaluation in immune cell-based therapies in living donor or deceased donor liver transplantation.
[1] Regulatory Dendritic Cells
[2] Cell Therapy
[3] Transplant tolerance
[4] Liver transplantation
[5] Immunosuppression/immune modulation
