Revised Siplizumab-based conditioning regimen for combined kidney and bone marrow transplantation improved mixed hematopoietic chimerism with significant enrichment of regulatory T cells
Ryo Otsuka1, Leonardo V Riella1, Thomas Spitzer1, Kerry Crisalli1, Gabriel Baysa1, Dana Naso1, Patrick Reeves1, A Benedict Cosimi1, Nahel Elias1, Tatsuo Kawai1.
1Legorreta Center for Clinical Transplant Tolerance, Massachusetts General Hospital, Boston, MA, United States
Introduction: Our previous clinical trials demonstrated that transient expansion of donor hematopoietic cell lineages through donor bone marrow transplantation with anti-CD2 mAb (Siplizumab)-based conditioning regimen can lead to long-term (>17 years) renal allograft tolerance in humans. While these results are promising, our initial conditioning regimen was consistently associated with transient acute kidney injury when chimerism rapidly disappeared around day 10-14 (chimerism transition syndrome; CTS). To overcome CTS, a revised conditioning regimen was evaluated in eight patients.
Method: Our previous conditioning regimen that included anti-CD2 mAb (Siplizumab), rituximab, cyclophosphamide (CY: 60 mg/kg x2), and thymic irradiation, was modified by adding low-dose fludarabine (Flu: 10 mg/m2 x3) while lowering the dose of CY (22.5 mg/kg x2) (Fig. 1). After combined kidney and bone marrow transplantation, patients were treated with tacrolimus monotherapy. After a biopsy at 6 months, tacrolimus is slowly tapered off over 12-24 months.
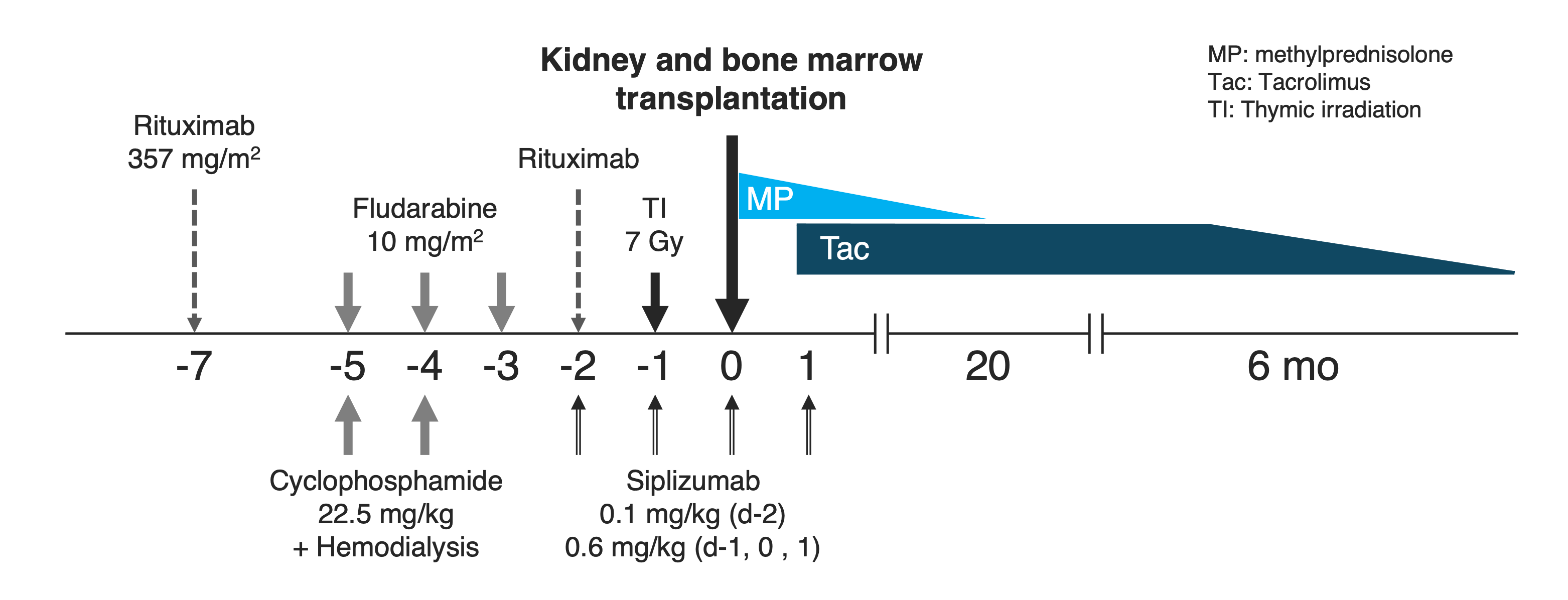
Results: Thus far, eight patients have participated in the study. Mixed hematopoietic chimerism was achieved in all patients without infectious complications or GVHD. CTS was not observed except for one patient (Patient #6) who developed CTS with elevated creatinine when chimerism was lost on day 14. This patient’s kidney function recovered to the baseline levels (serum creatinine 1.4) within 2 months. Kidney function of all other patients has been normal (serum creatinine 1.4±0.2 mg/dl) with tacrolimus monotherapy. Markedly enriched regulatory T cells (Tregs; CD4+CD25+CD127lowFOXP3+) were consistently observed for 6 months after transplantation (Fig. 2). Tapering of tacrolimus monotherapy in these patients is in progress. MLR in a patient who achieved stable kidney function with undetectable tacrolimus, revealed donor-specific expansion of regulatory T cells along with increased expression of HLA-DR.
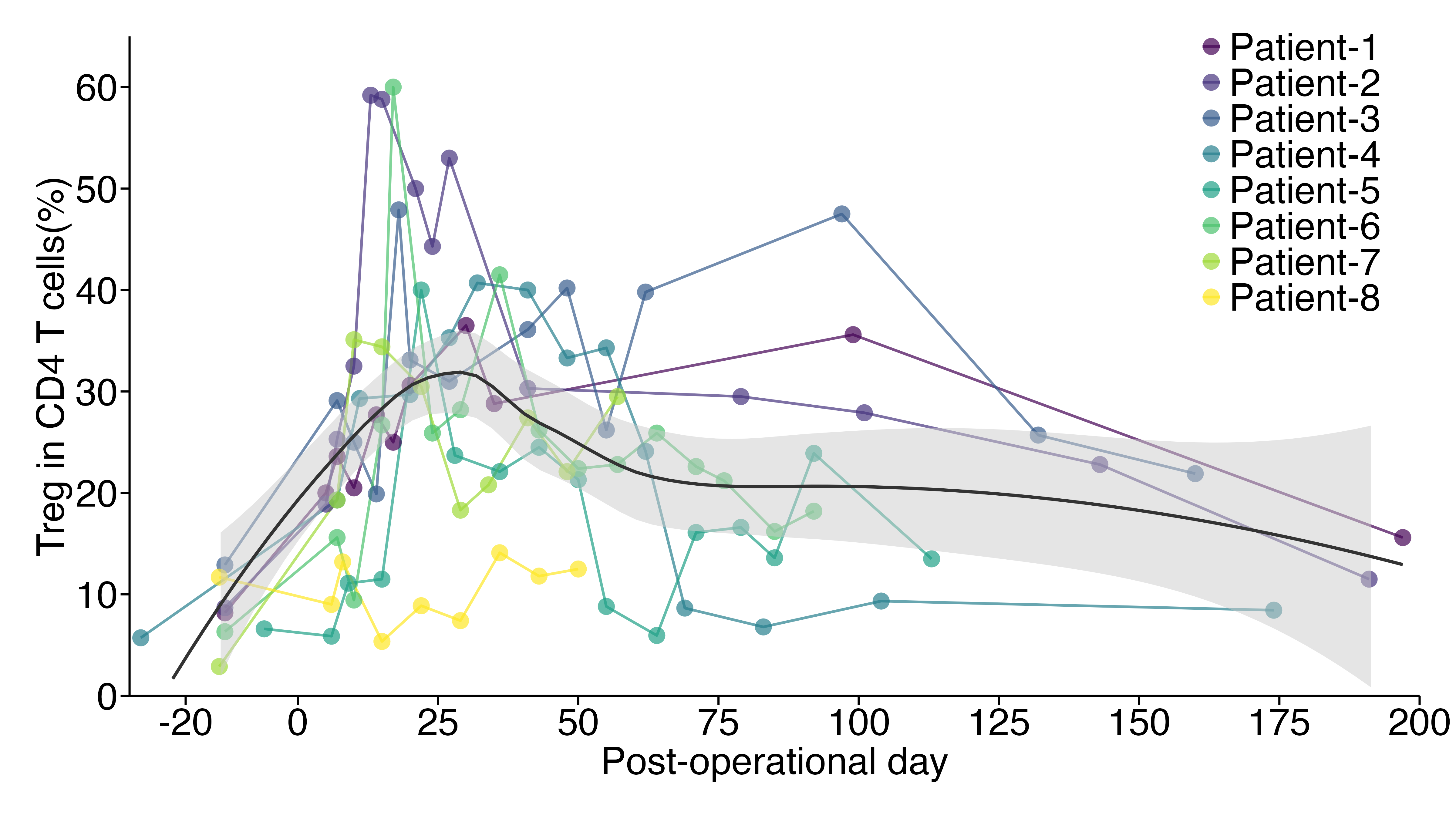
Conclusion: A modified preconditioning regimen has successfully overcome CTS with improved mixed transient hematopoietic chimerism. Markedly enriched Tregs have been observed long-term after CKBMT.
[1] Mixed Chimerism
[2] Kidney Transplantation
[3] Tolerance Induction
[4] Bone Marrow Transplantation
