Muhammed Esad Gunes, United States has been granted the TTS Basic and Translational Mentee-Mentor Award
MHC class II sharing promotes donor treg-mediated tolerance after intestinal transplantation in swine
Muhammed Esad Gunes1, Satyajit Patwardhan1, Sarah Merl1,2, Rebecca Jones1,3, Mercedes Martinez1,4, Greg Nowak1,5, David H Sachs1,6,7, Tomoaki Kato1,7, Megan Sykes1,7,8, Joshua I Weiner1,7.
1Columbia Center for Translational Immunology, Columbia University, New York, NY, United States; 2Department of Pathology, Columbia University, New York, NY, United States; 3Graduate School, Northwestern University, Chicago, IL, United States; 4Department of Pediatrics, Columbia University, New York, NY, United States; 5Division of Transplantation Surgery, Karolinska Institute, Stockholm, Sweden; 6Department of Surgery, Harvard University, Boston, MA, United States; 7Department of Surgery, Columbia University, New York, NY, United States; 8Department of Microbiology & Immunology, Columbia University, New York, NY, United States
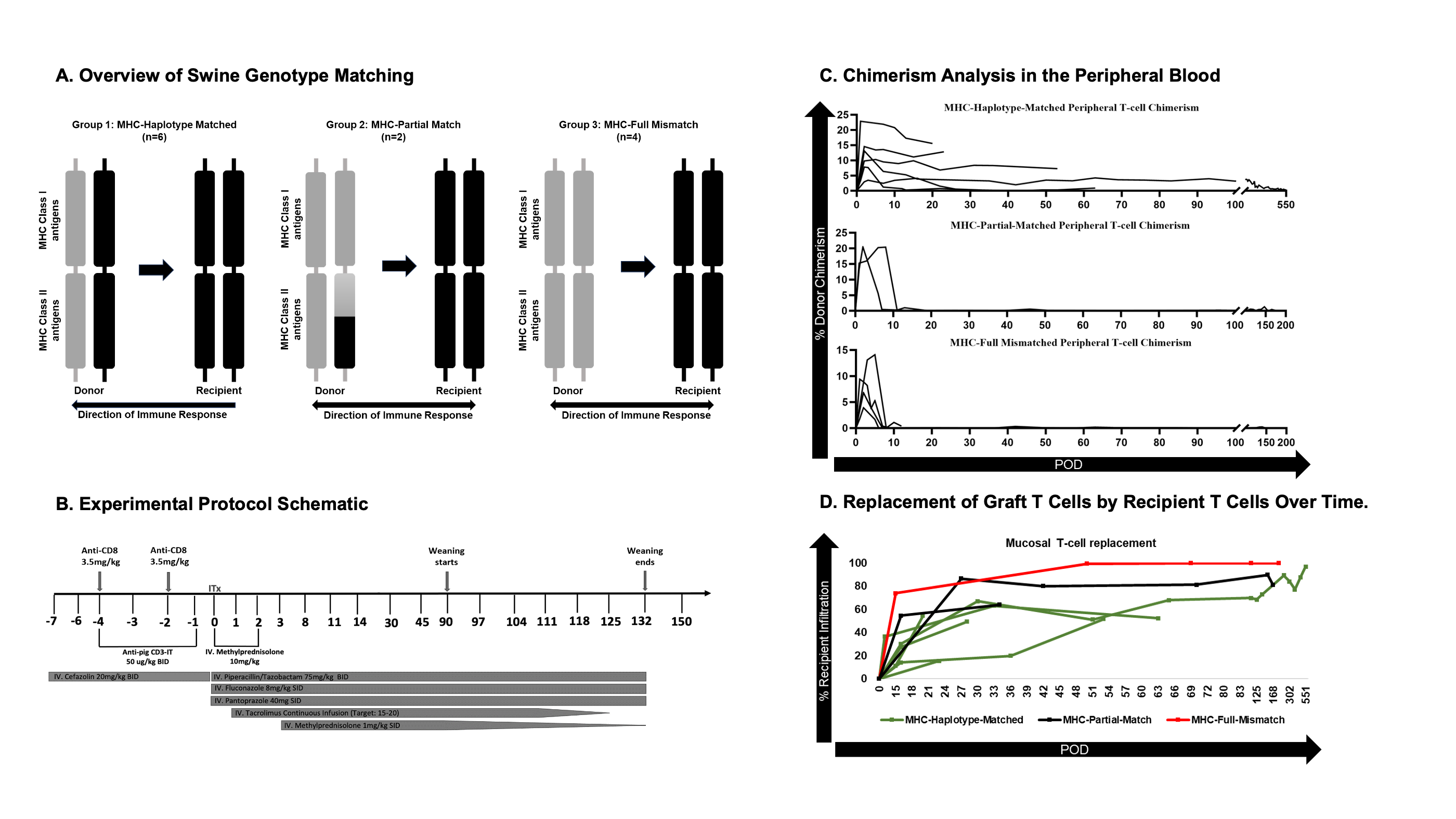
Introduction: Intestinal transplantation (ITx) is the definitive treatment for patients with intestinal failure, but high rates of rejection and complications from immunosuppression (ISP) limit its utility. The development of transplantation tolerance could address these problems. Furthermore, we developed an ITx model in miniature swine with defined MHC haplotypes to evaluate chimerism and immune responses in various clinically relevant scenarios.
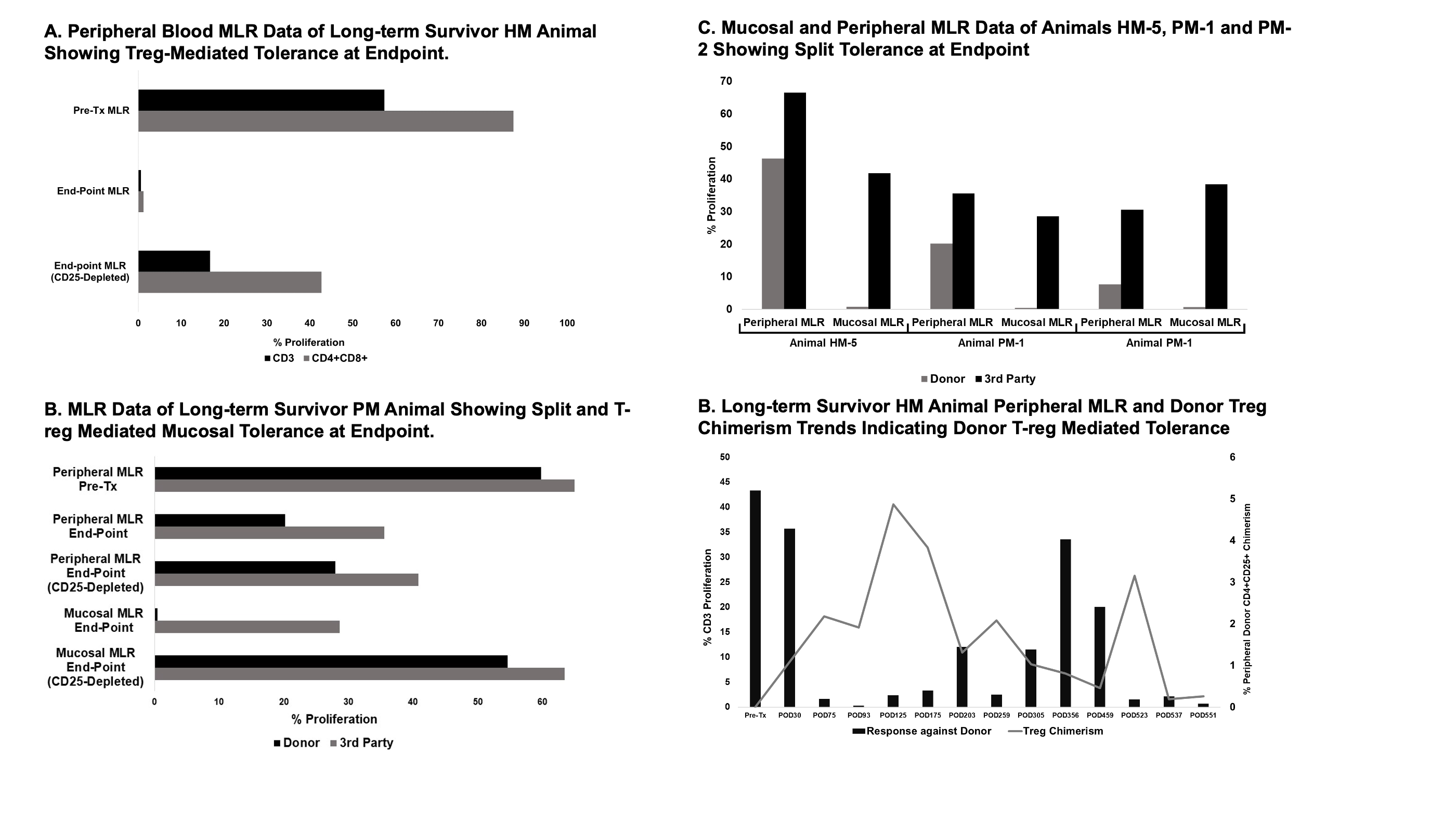
Methods: We performed orthotopic ITx between MHC haplotype-matched (HM, n=6), full-mismatched (FM, n=4), and partial-matched (PM, having Class II alleles with marked overlap, n=2) pairs. ISP mirrored our clinical protocol with T-cell depletion, steroids, and tacrolimus. ISP was weaned off after day 90. Peripheral, mucosal, and bone marrow chimerism were tracked weekly, biweekly, and monthly, respectively. Endoscopic biopsies were collected biweekly and evaluated for rejection. Donor-specific antibody (DSA) assays were done before and 45 days after transplant. Cellular immunity was monitored by mixed lymphocyte reaction assays (MLR) with and without regulatory T cell (Treg) depletion (with anti-CD25 antibody), including a novel mucosal application of this assay.
Results: Of the 6 HM recipients, one developed GVHD, and the remaining 5 showed no clinical or histopathological signs of rejection, with durable multilineage chimerism in the peripheral blood, slow replacement of donor graft T cells by the recipient, and Treg-mediated donor-specific hyporesponsiveness (DSH) in blood and graft mucosa, demonstrated by MLR. One survived over 420 days after weaning ISP. Donor Treg trends indicated that peripheral DSH in HM animals was mediated by donor Tregs. All 4 FM recipients lost initial blood chimerism, developed DSA, had no DSH, and rejected their grafts. Both PM recipients lost peripheral blood chimerism without the development of de-novo DSA and developed a previously undescribed form of “split tolerance” (local tolerance in the graft but not blood). One survived until day 174 after weaning ISP without any signs of rejection. Treg depletion suggested that mucosal DSH in PM animals was mediated by donor Tregs.
Conclusion: We developed a swine ITx model that permitted durable mixed chimerism and tolerance induction. Our novel Treg-depletion mucosal MLR assay suggested that the extent of donor Treg-mediated tolerance correlated with the degree of MHC Class II sharing. While the FM animals lacked tolerance in both blood and graft, the PM animals developed local graft tolerance mediated by donor Tregs, which, with increasing Class II sharing in the HM animals, additionally migrated to the periphery and promoted DSH and durable mixed chimerism in the blood. Further study should evaluate whether Class II matching has similar utility in human ITx recipients.
This research was supported by the NIH grant R01AI138547 and the Irving Institute for Clinical and Translational Research at Columbia University. Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health, under the award S10OD030282.
[1] Intestinal Transplant
[2] Mixed Chimerism
[3] Tolerance
[4] Mucosal Tolerance
[5] Split Tolerance
[6] Treg
[7] Local Tolerance
