The mechanism research of intrahepatic glutamine metabolism promoting the differentiation of GARP Tregs to reshape the immune tolerance microenvironment post liver transplantation
Jian Gu1, Yu Li1, Qiuyang Cheng1, Ling Lu1.
1Jiangsu People's Hospital, Jiangsu People's Hospital, Nanjing, People's Republic of China
Objectives:This study aims to explore the intrinsic differences in metabolic profiles and immune characteristics of allografts between immune-tolerant and immune-stable states after liver transplantation. and seeks to comprehend the mechanisms, roles, and significance of GARP+Tregs in the formation of immune tolerance after liver transplantation.
Methods:This study enrolls liver transplant recipients who have stable liver function while on medication, as well as patients who have maintained immunological tolerance for over one year without medication. We aim to explore the underlying mechanisms through analysing metabolomic and transcriptomic sequencing. Additionally, GARPFL/FLFoxp3cre mouse will be established and utilized in a liver transplant mouse model to validate the findings, thereby assessing the role of GARP+Tregs in immune tolerance after liver transplantation.
Results: This study uses single-cell sequencing technology to discover the enrichment of GARP+Tregs within the transplanted liver of patients exhibiting immune tolerance after liver transplantation. These Tregs exhibited significantly enhanced arginase cycle metabolism activity, which correlated with increased glutamine decomposition and ammonia concentration within the transplanted liver of immune-tolerant patients after transplantation. Similar findings were validated in a mouse liver transplant model.
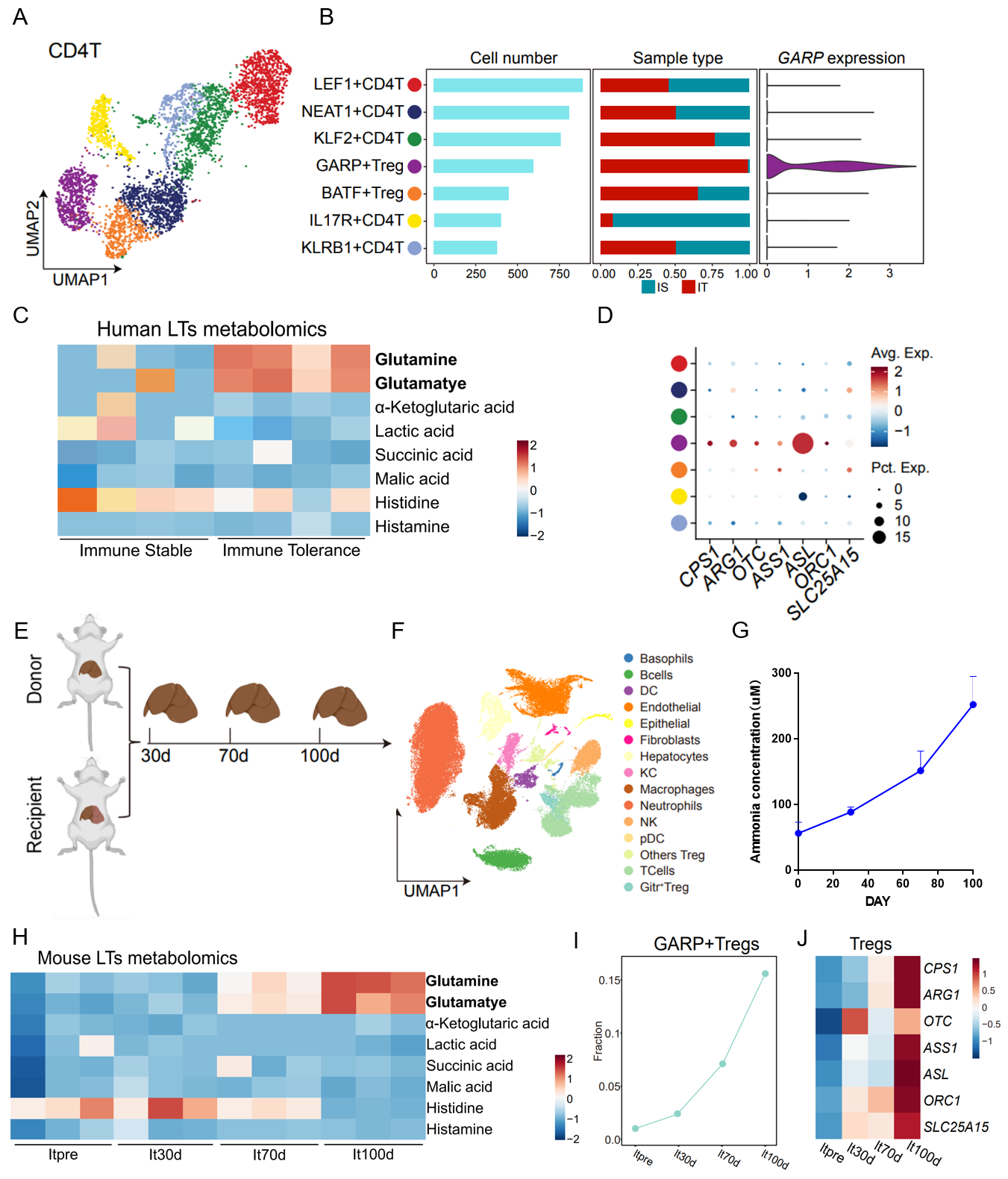
Since glutamine decomposition results in ammonia accumulation within the organism, this study also treated various types of T cells with ammonia in vitro. Results revealed that ammonia induced apoptosis in various T cell subsets; however, due to the enhanced arginase activity of GARP+Tregs, they were capable of decomposing and utilizing ammonia to enhance their own immunosuppressive function.
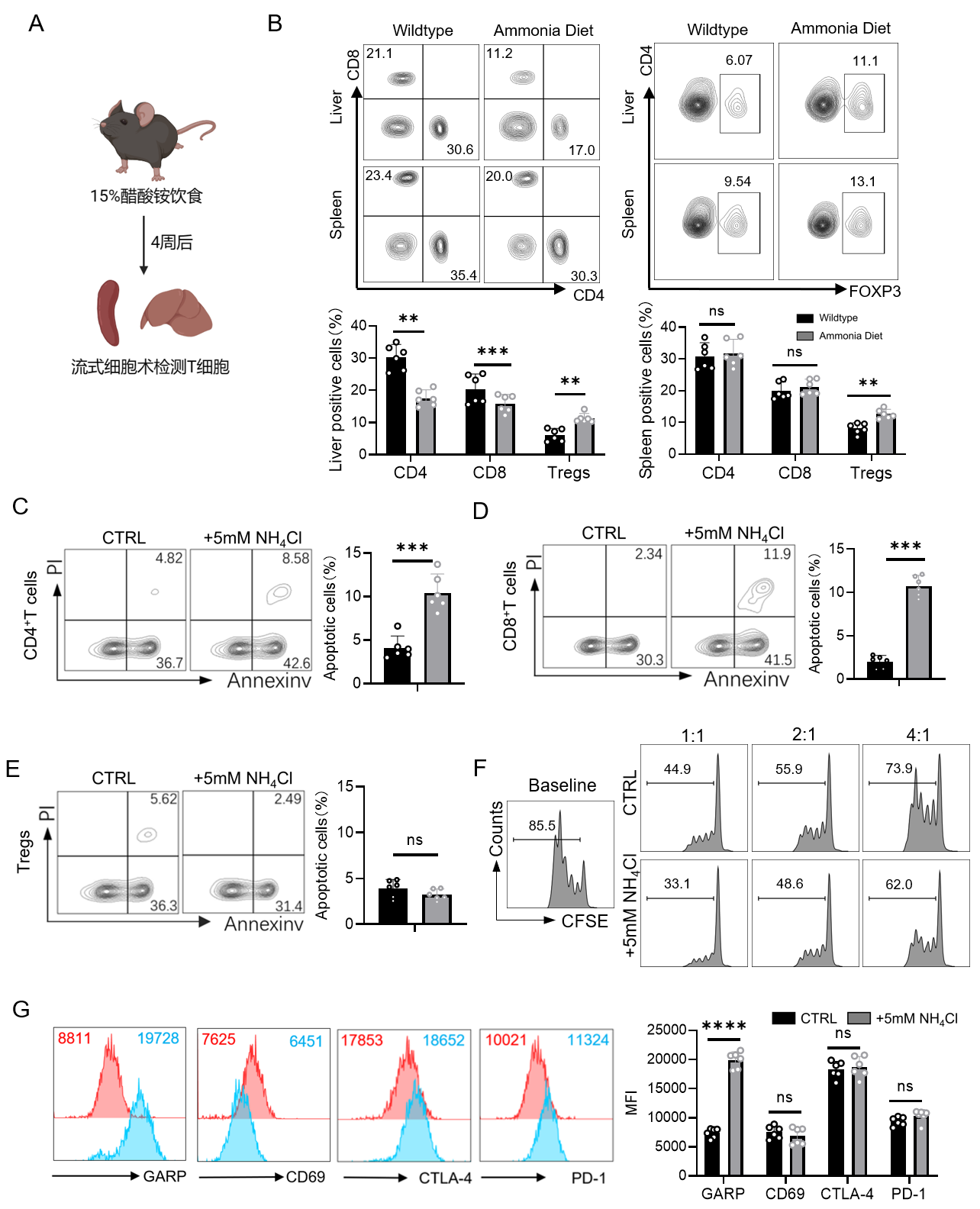
Additionally, this study finds that ammonia can bind to SRC3, highly expressed in Tregs, enhancing its interaction with STAT3, ultimately promoting STAT3 phosphorylation and ASL transcription.
Conclusion:The decomposition of glutamine and accumulation of ammonia within the transplanted liver play crucial roles in the microenvironment of immune tolerance after liver transplantation. Ammonia can induce apoptosis of immune cells within the transplanted liver. However, Tregs perceive ammonia through the SRC3-STAT3 complex, initiating arginase cycle metabolism to decompose and utilize ammonia, thereby enhancing their immunosuppressive function. Ultimately, reshaping the immune microenvironment through the inhibition of effector T cells and enhancement of Tregs achieves metabolic modulation and facilitates the establishment of clinical immune tolerance after liver transplantation.
