Alpha-1 antitrypsin-loaded nanoparticles improve transplantation outcomes in an acute liver failure rat model
Giuseppe Pettinato1, Matthew Massaad1, Wayne Hawthorne2, Xuejun Wen3, Robert A Fisher1.
1Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States; 2Surgery, Westmead Hospital, University of Sydney, Westmead, Australia; 3Chemical and Life Science Engineering, Virginia Commonwealth University, Richmond, VA, United States
Introduction: Advanced liver disease signifies a potentially life-threatening condition often treated with liver transplantation, which uniquely doesn't require histocompatibility antigen testing. Despite improvements, liver transplantation still carries perioperative risks and necessitates lifelong immunosuppression, with a significant risk of immune rejection. Immune rejection involves complex interactions between hepatic and immune cells, primarily mediated by T cells recognizing alloantigens. To address immunosuppressive drug toxicity and donor scarcity, alternative solutions are being explored such as artificial liver replacement, including the use of human induced pluripotent stem cells (hiPSCs) for liver organoid generation. Our research focuses on mitigating acute inflammatory responses post-transplantation by incorporating alpha-1 antitrypsin (AAT)-loaded nanoparticles within our liver organoids. This novel approach aims at liver regeneration/replacement while avoiding the use chronic systemic anti-inflammatory drug use.
Method: AAT-loaded nanoparticles were generated using a dextran-based method and were interspersed within the hiPSCs during hEB formation by using our agarose micromold technology. After liver differentiation using our protocol, liver organoids containing AAT-loaded nanoparticles (AAT-NP) were tested in vitro using a pro-inflammatory cytokine cocktail (TNF-α + IL-1β + INF-γ called Cytomix) and then tested for their viability and function by performing annexin V and PI staining assays to evaluate the number of viable, apoptotic cells within the organoids. Liver organoids containing AAT-NP were then used in an in vivo acute liver failure (ALT) rat model to test their ability to protect from post-transplantation inflammation. Human albumin in the rat serum was measured to assess engraftment, and overall viability was compared between the treated and control rats.
Results: In vitro treatment with Cytomix of our liver organoids containing AAT-NP showed great improvement in organoids viability compared to control (organoids without AAT-NP). Their gene and enzymatic functions were also preserved compared to the control (Fig 1). In vivo transplantation of AAT-NP liver organoids demonstrated a substantial improvement in the survival rate (75% for AAT-NP VS 62.5% for CRTL), as well as, sustained albumin secretion (mean albumin levels were 21.4 ± 3.08 ng/mL for AAT-NP VS 10.2 ± 1.02 ng/mL for CRTL) (Table 1).
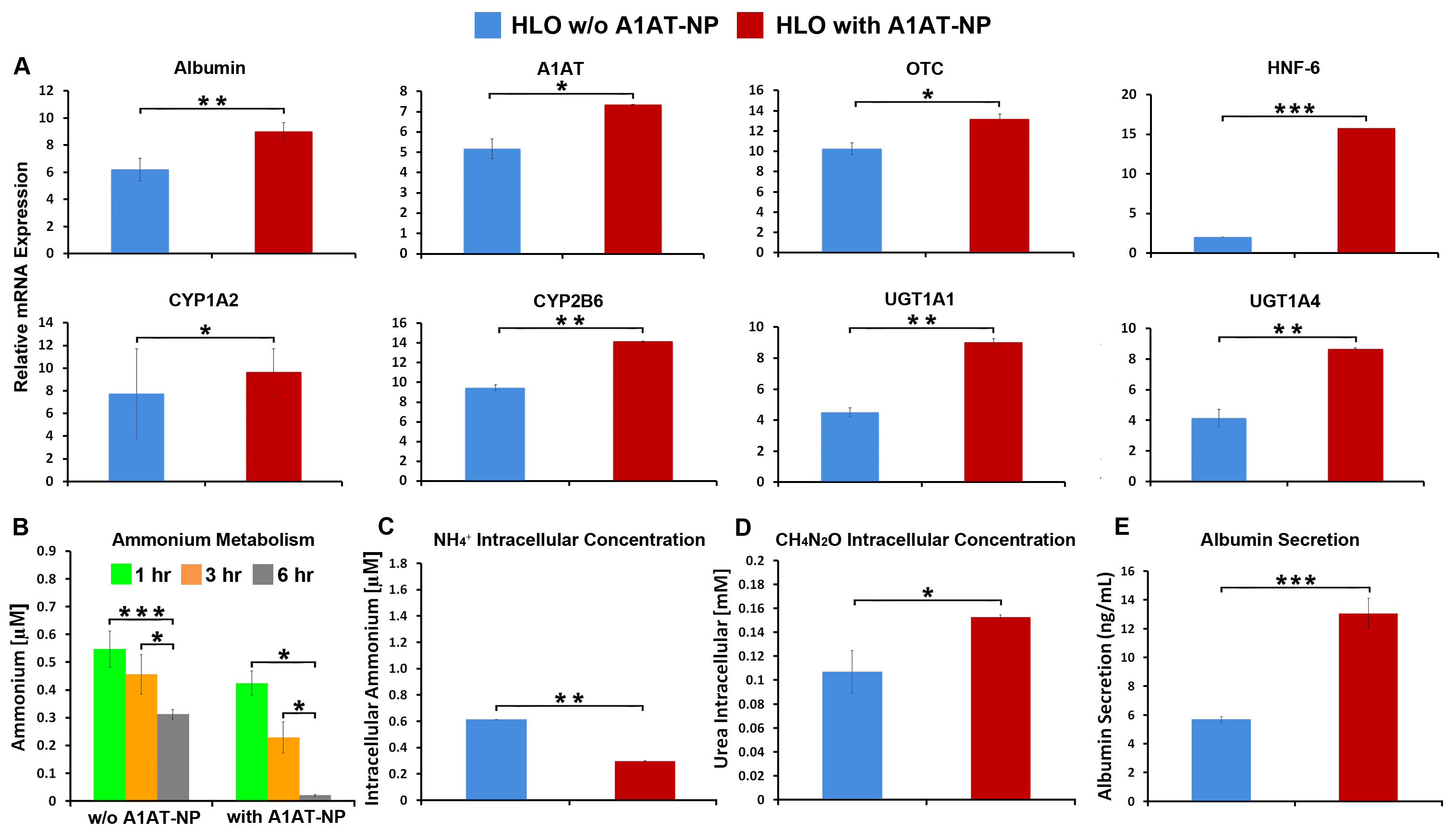

Conclusion: The use of AAT-NP interlaced within liver organoids demonstrated a protective effect against in vitro cytokine insult, as demonstrated by the overall viability and liver function. In an in vivo setting the presence of AAT-NP within the liver organoids showed an ability to improve engraftment and reduce post-transplantation inflammation as confirmed by the prolonged survival and significantly sustained human albumin secretion in the rat’s blood.
We thank CSL Behring for providing the funding for this project.
[1] Liver Organoids; Regenerative Medicine; Alpha-1 Antitrypsin, A1AT, Immune regulation
