Fatma Dogan, United States has been granted the TTS Scientific Congress Award
Immunoisolation and islet survival in the absence of systemic immune suppression enabled by co-delivery of CXCL12 and FasL containing microgels in allo-islet transplanted diabetic mice
Fatma Dogan1, Esin Ozkan1, Aaruni Arora1, Katie Lu1, Esma S Yolcu2,3, Angelica Torres4, Haval Shirwan2, Andrés J García4, Timothy Brauns1, Mark C Poznansky1.
1Vaccine and Immunotherapy Center, Massachusetts General Hospital,Harvard Medical School, Boston, MA, United States; 2Department of Pediatrics, Ellis Fischel Cancer Center, School of Medicine, University of Missouri, Columbia, MO, United States; 3NextGen Precision Health Institute, Ellis Fischel Cancer Center, School of Medicine, University of Missouri, Columbia, MO, United States; 4Petit Institute for Bioengineering and Bioscience, Woodruff School of Mechanical Engineering, Georgia Institute of Technology, Atlanta, GA, United States
Background: Type 1 diabetes (T1D) is a chronic disease characterized by the autoimmune destruction of insulin-producing β-cells in the pancreas. Replacement of β-cells by islet transplantation has proven to be successful in providing a functional cure for T1D. We have previously shown that incorporation of the chemokine, CXCL12 promotes β-cell function, viability, and regeneration, provides immune protection and facilitates vascularization at the graft site that enhances islet function in the context of islet transplantation in both small and large animal models. In addition, streptavidin Fas Ligand (SA-FasL) microgels induce immune acceptance of allogeneic islets through the expansion of T regulatory cells (Tregs).
Objective: This study evaluates two complementary approaches for extending islet graft survival in a mouse model of T1D through the co-delivery of CXCL12 and Fas-Ligand (FasL) within microgels in an allogeneic transplant setting. Both approaches enhance islet function and provide immunoprotection in both small and large animal models through distinct immunomodulatory mechanisms. We hypothesized that combining these factors in microgels would create a synergistic immunoisolation effect at the graft site, increasing regulatory T cell numbers while repelling effector cytotoxic T cells from the graft site.
Methods: The microgels were generated using PEG-4MAL macromers at defined size and composition and biotinylated microgels were used to capture SA-FasL or SA-CXCL12. Fluorescence imaging was conducted to validate successful engineering of microgels with SA-CXCL12 and SA-FasL fusion proteins. In vitro release of CXCL12 from the microgel was tested. A total of 2400 engineered microgels and 1200 unmodified BALB/c islets were co-transplanted into the epididymal-fat-pad (EFP) of streptozotocin (STZ)-treated diabetic C57BL/6 mouse recipients.
Results: Bioactivity of SA-CXCL12 as a chemotactic and fugetactic agent was demonstrated using splenocytes and primary CD3+T cells in vitro.
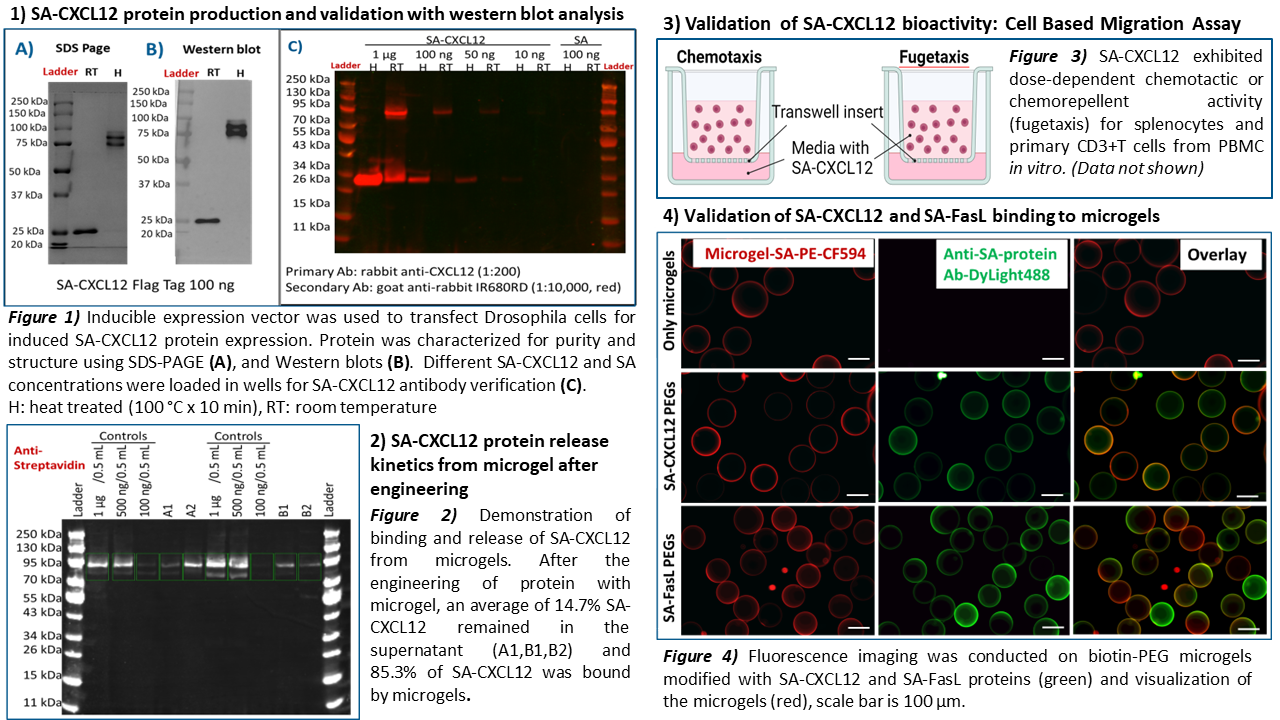
Our transplant results demonstrated that microgels loaded with CXCL12 and FasL, when co-transplanted with allogeneic islets, achieve prolonged diabetes reversal in STZ-treated mice without systemic immunosuppression compared to either agent alone. The mice group receiving combination therapy successfully maintained normoglycemia for up to 42 days without the need for systemic immunosuppression (*** p< 0.001).
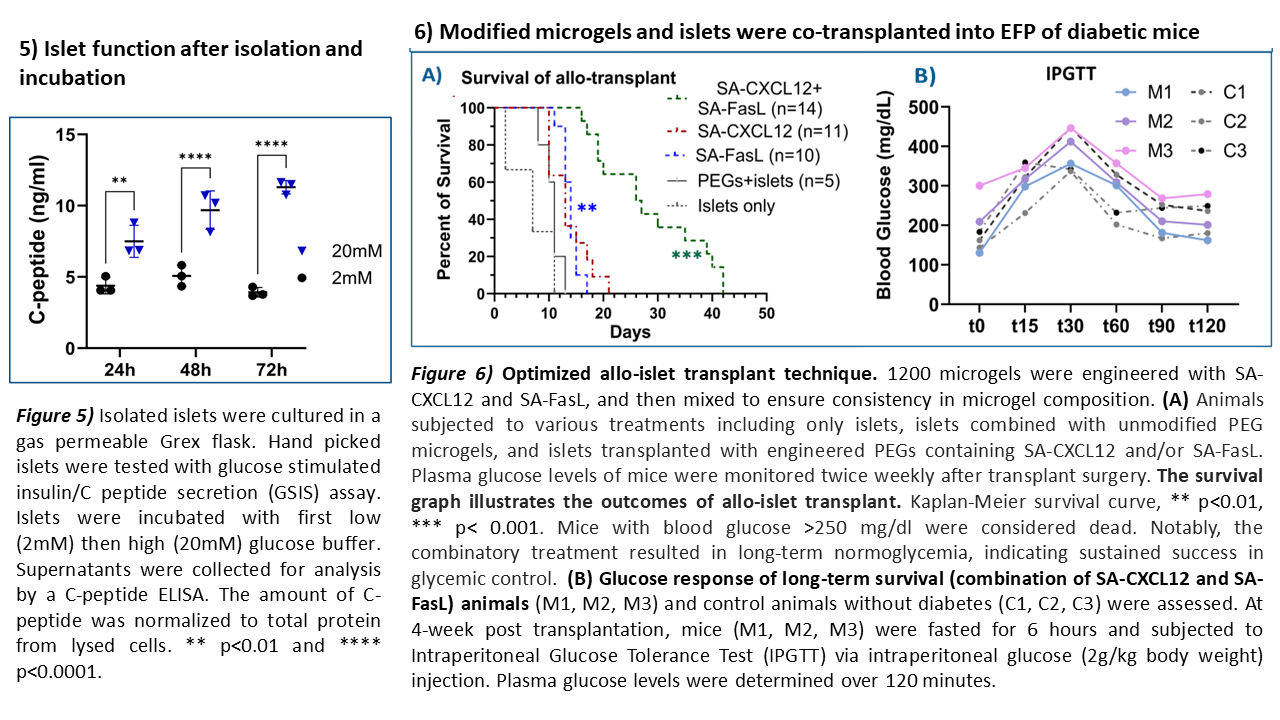
Conclusion: Allo-islet transplantation of combined SA-CXCL12 and SA-FasL engineered microgels with islets was successfully optimized. This study lays the groundwork for further preclinical investigation in the NOD/LtJ mouse model of autoimmune diabetes.
Next steps: Assessment of immune protection (Treg / Effector CD8+ T cell subpopulations) in experimental and control groups along with immune phenotyping of immune cells in lymph node and spleen. Assessment of infiltrating immune cell populations at the graft site (EFP) of experimental and control animals.
This study was funded by the Juvenile Diabetes Research Foundation (JDRF) and the VIC Innovation Fund.
[1] Type 1 Diabetes, Allograft Survival, Islet transplant, microgels, SA-CXCL12, SA-FasL, immunoprotection
