Moritz Muckenhuber, Austria has been granted the TTS Basic and Translational Mentee-Mentor Award
Interleukin-6 blockade prevents costimulation blockade-resistant allograft rejection by promoting intragraft regulation
Moritz Muckenhuber1, Konstantinos Mengrelis1, Anna M Weijler1, Romy Steiner1, Verena Kainz1, Marlena Buresch1, Heinz Regele2, Sophia Derdak3, Anna Kubetz1, Thomas Wekerle1.
1Division of Transplantation, Medical University of Vienna, Vienna, Austria; 2Clinical Institute of Patholoogy, Medical University of Vienna, Vienna, Austria; 3Core Facilities, Medical University of Vienna, Vienna, Austria
Introduction: Current induction therapies, including anti-thymocyte globulin (ATG), fail to prevent costimulation blockade-resistant allograft rejection. Herein, employing a mouse model of cardiac transplantation, we investigated the mechanisms why ATG fails to prevent rejection under CTLA4-Ig and how this barrier can be overcome.
Methods: Female C57BL/6 mice were grafted with a fully mismatched BALB/c cardiac allograft and were treated with CTLA4-Ig maintenance therapy (0.25mg/dose on days 0, 4, 14, 28, 56, 84). Groups received additional ATG induction (0.15mg on days 0 and 5) with or without interleukin-6 (IL6) blockade (anti-IL6 mAb [αIL6], 0.6mg on day -1; 0.3mg on days 3 and 6). Where indicated, Tregs were depleted using an anti-CD25 mAb (clone: PC61; 0.25mg/dose) either before (day -5 and -2) or 4 weeks after (days 28 and 35) transplantation. Spleen and graft infiltrating leukocytes were analyzed using flow cytometry. Heart allograft survival was followed for 100 days and grafts were assessed. Bulk RNA sequencing of cardiac allografts and isolated graft-infiltrating leukocytes was performed.
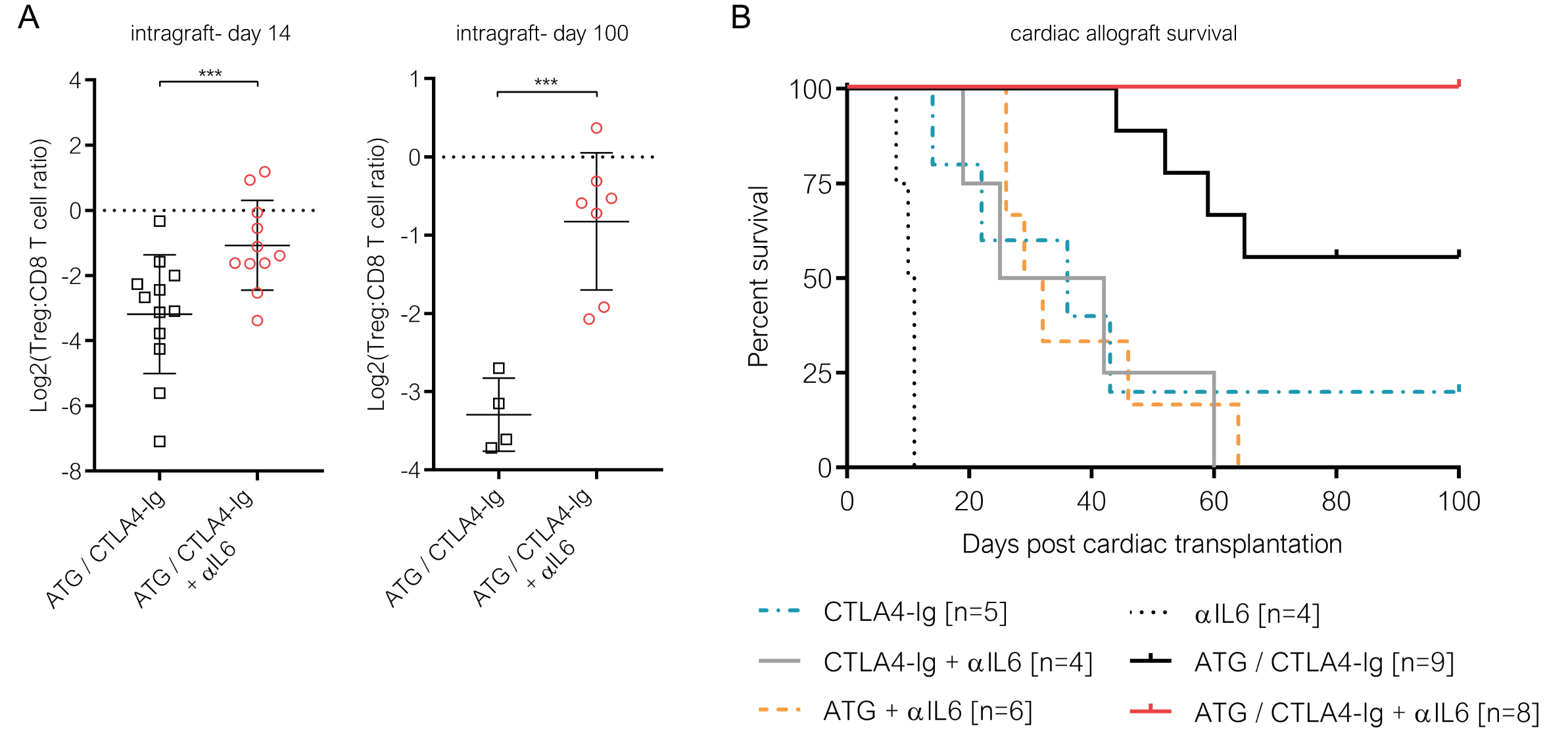
Results: ATG prolonged survival but failed to prevent rejection under CTLA4-Ig (ATG/CTLA4-Ig), with 50% of the grafts being lost during the 100-day follow up. While ATG-mediated depletion shifted the balance between Tregs and effector T cells in favor of Tregs in the peripheral lymphoid compartment, it failed to do so within cardiac allografts. Furthermore, ATG triggered the secretion of pro-inflammatory cytokines, including IL6. Neutralizing IL6 in mice treated with CTLA4-Ig and ATG alleviated graft inflammation and allowed Tregs to accumulate within cardiac allografts (Figure 1A). Thereby αIL6 resolved the imbalance between the peripheral and intragraft compartment that we observed following ATG induction. RNA sequencing revealed a shift in signaling patterns within graft infiltrating leukocytes towards Th2-associated cytokines, including interleukin-10, in recipients treated with additional IL-6 blockade. Thereby, neutralizing IL-6, together with ATG induction, allowed CTLA4-Ig to achieve 100% (8/8) long-term cardiac allograft survival (Figure 1B). This benefit was abrogated when Tregs were depleted before or 4 weeks after transplantation, indicating that the effect of αIL6 in combination with CTLA4-Ig/ATG is Treg-dependent.
Conclusion: Our data reveal that ATG contributes to a pro-inflammatory microenvironment that impedes intragraft regulation in CTLA4I-Ig treated recipients. IL-6 blockade prevents costimulation blockade-resistant rejection in a Treg-dependent manner and provides a clinically testable approach for overcoming higher rejection frequencies seen in ATG/belatacept-treated transplant recipients.
[1] costimulation blockade
[2] regulatory T cells
[3] regulation
[4] anti thymocyte globulin
[5] interleukin-6
