
Cell-free DNA testing – A new arrow in the ID doctor’s quiver? Or a stewardship nightmare?
Cameron Wolfe1, Alejandro Antonia1, Manuela Carugati1, Madeleine R Heldman1, Jonathan Huggins1, Julia A Messina1, Eileen K Maziarz1, Jennifer H Saullo1, Ilan S Schwartz1, Julie M Steinbrink1, Patrick CK Tam1, Arthur W Baker1.
1Infectious Diseases, Duke University Medical Center, Durham, NC, United States
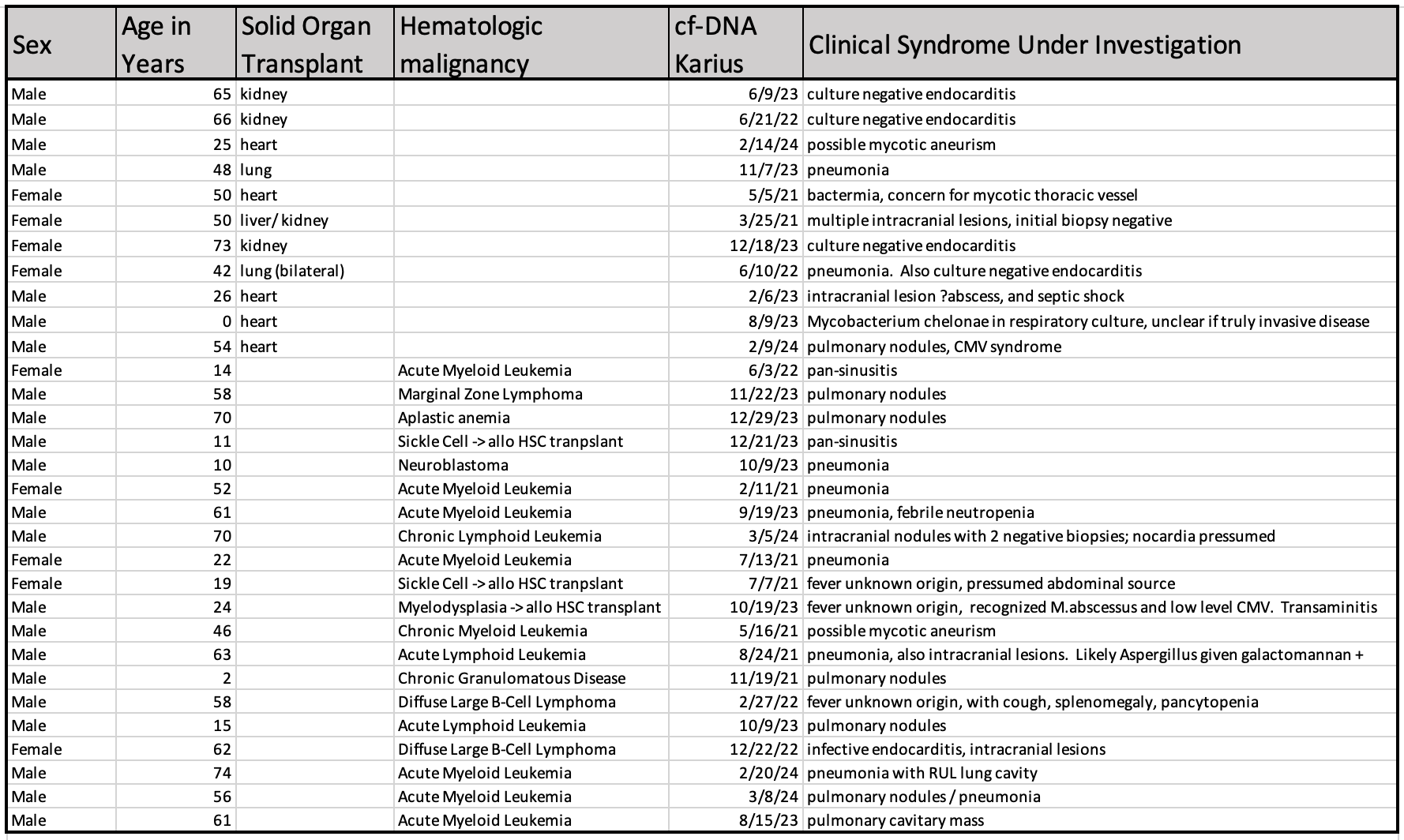
Introduction: Cell-free DNA (cf-DNA) testing of plasma offers new opportunities for broad DNA-based pathogen discovery and monitoring in transplant patients. Yet best practices models to guide when to perform such tests are lacking; testing is expensive (approx. US $1500 per test), and some microorganisms detected solely by plasma cf-DNA are of uncertain significance . We sought to evaluate the use of a cf-DNA platform (Karius) at a single large transplant program, comparing solid organ transplant (SOT) patients against those with hematologic malignancies (HM).
Method: We performed a retrospective analysis of patients with cf-DNA testing, between Jan 1, 2021, until March 10, 2024, at Duke University Medical Center. Patient demographics, underlying disease, and reasons for cf-DNA testing were collated. We reviewed preexisting microbiologic diagnoses and compared cf-DNA results and consequent management strategies to determine whether actionable data resulted from the test.
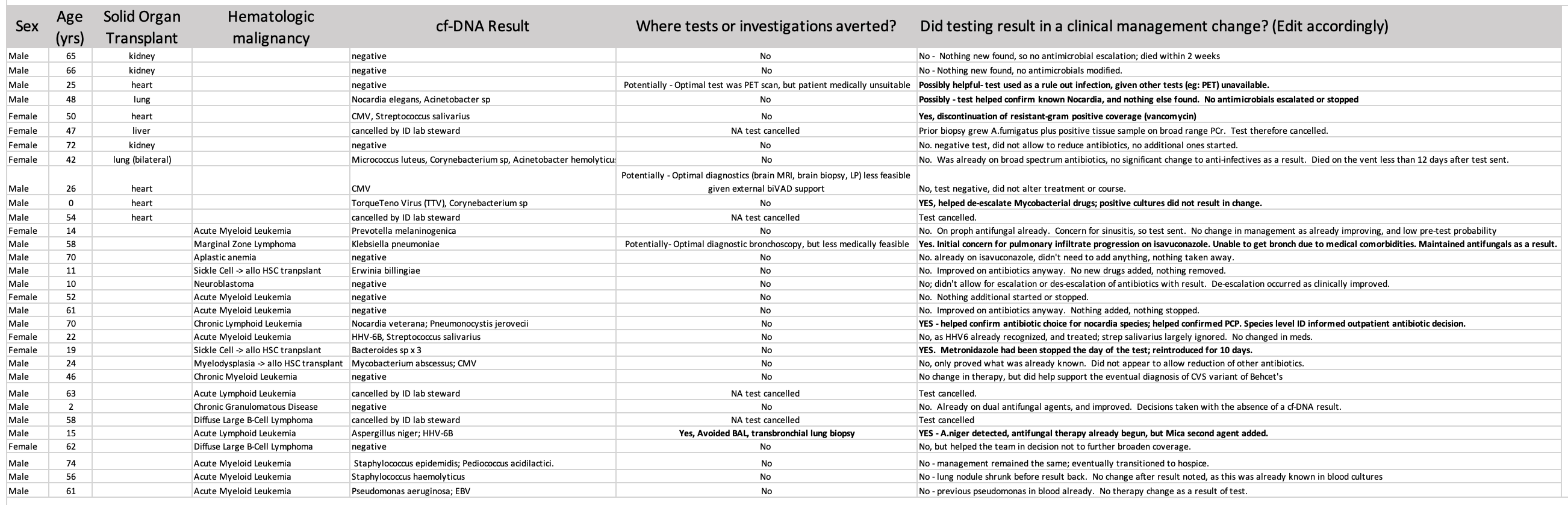
Results: Thirty one underwent plasma cf-DNA testing; 11 solid organ transplant patients and 20 with hematologic malignancy (fig 1). Median age was 50yrs, IQR 22-62yrs, 9 (29%) female. TThe most common indication for cf-DNA testing was in the evaluation of radiological pulmonary nodules or clinical pneumonia (17 cases) followed by presumed endovascular infections (8 cases) and intracranial lesions (5 cases). Bacteria were identified in 12 cases, herpesvirus in 7, nocardia or mycobacteria in 3 patients, and fungal pathogens in 2 (fig 2). Eleven of 27 tests did not detect any DNA pathogens. Four tests were cancelled by laboratory stewardship.
In only 2 patients did cf-DNA testing result in impactful additions to clinical management; anaerobic antibiotics were continued after multiple Bacteroides species were noted inone case and detection of A. niger allowed for dual antifungal therapy to be initiated a second. Negative cf-DNA tests lent support for no further antibiotic escalation in 5 cases, and allowed for de-escalation in 3 (respectively anti-mycobacterials, drug-resistant gram negative agents, and antifungals). In one case, bronchoalveolar lavage and lung biopsy was avoided as a result of the cf-DNA testing.
In 5 cases, cf-DNA analysis detected microorganisms diagnosed through routine laboratory testing. No trends were observed when comparing the clinical utility of cf-DNA testing in SOT patients versus those with a HM.
Conclusion: Plasma cf-DNA did not lead to immediate antimicrobial changes in the majority of transplant patients. However, the impact of cf-DNA testing on clinical decision making beyond antimicrobial management requires additional evaluation. Stewardship programs should consider the indirect costs of medical interventions that are avoided or initiated based on plasma cf-DNA results when evaluating the test’s cost effectiveness.
[1] infection
[2] cell-free DNA
[3] antimicrobial
[4] laboratory stewardship
