Delineating the role of biological sex in organ transplantation: Identifying estrogen receptor α enhanced 4-1BB signaling as a critical component of T cell-mediated alloimmunity
Yao Xiao1, Keita Nakamori1, Tomohisa Matsunaga1, Friederike Martin1, Merih Gizlenci1, Elizabeth Zicari1, Reza Abdi2, Yuko Sato1, Hao Zhou1, Stefan Tullius1.
11Division of Transplant Surgery, Transplant Surgery Research Laboratory, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States; 2Transplantation Research Center, Renal Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
Purpose: While the impact of biological sex on autoimmune diseases, infections, and malignancies is broadly recognized, effects on alloimmunity and transplant outcomes impacted by hormonal, genetic, and epigenetic components have been less well defined. Clinical data have shown that both age and biological sex affect transplant outcomes with inferior graft survival in young and superior outcomes in old female recipients, suggesting sex hormone-driven components of alloimmunity. Here, we present a novel mechanistic concept of estrogen receptor (ER)-mediated 4-1BB costimulatory T cell signaling within the allograft impacting transplant outcomes.
Methods and Results: To delineate the role of estrogen receptor (ER) on T cells, we generated T cell-specific ERα knockout (T-ERα KO) mice by crossing CD4Cre×ERαfl/fl C57BL/6 mice. Female T-ERα KO mice (2-3 months, n=5) and littermate wildtype control (WT) received fully MHC-mismatched skin or heart transplants from male DBA/2 mice. Graft survival was compared by Kaplan-Meier analysis. Allografts and spleens were procured by day 5 assessing graft morphology. Alloimmune responses were assessed intragraft, systemically and by mixed lymphocyte reaction (MLR).
T cell-specific ERα deficiency prolonged cardiac allograft survival significantly (p<0.05) with improved allograft morphology and reduced cellular infiltrates intragraft. T-ERα KO recipients demonstrated decreased amounts of graft infiltrating effector CD4+ T cells, IFNγ+CD4+ and CD8+ T cells, while IL4+CD4+ T cells increased (all p<0.05), indicating a less robust T cell response. ScRNAseq analysis of graft infiltrating immune cells identified an estrogen-dependent, attenuated activation of the 4-1BB costimulatory pathway in T cells. A significant reduction of 4-1BB expression on both effector CD4+ and CD8+ T cells from T-ERα KO recipients (both p < 0.05) confirmed that this pathway is regulated by ERα. In support, MLR revealed a reduced 4-1BB expression and inhibited activation of CD4+ T cells from T-ERα KO mice (both p < 0.01). Next, we treated WT and KO transplant recipients with an anti-4-1BB antagonist and observed a compromised immunosuppressive efficiency in T-ERα KO mice receiving skin or heart transplants (Figure 1), suggesting that anti-4-1BB immunosuppressive treatment is dependent on ERα-mediated signaling in T cells.
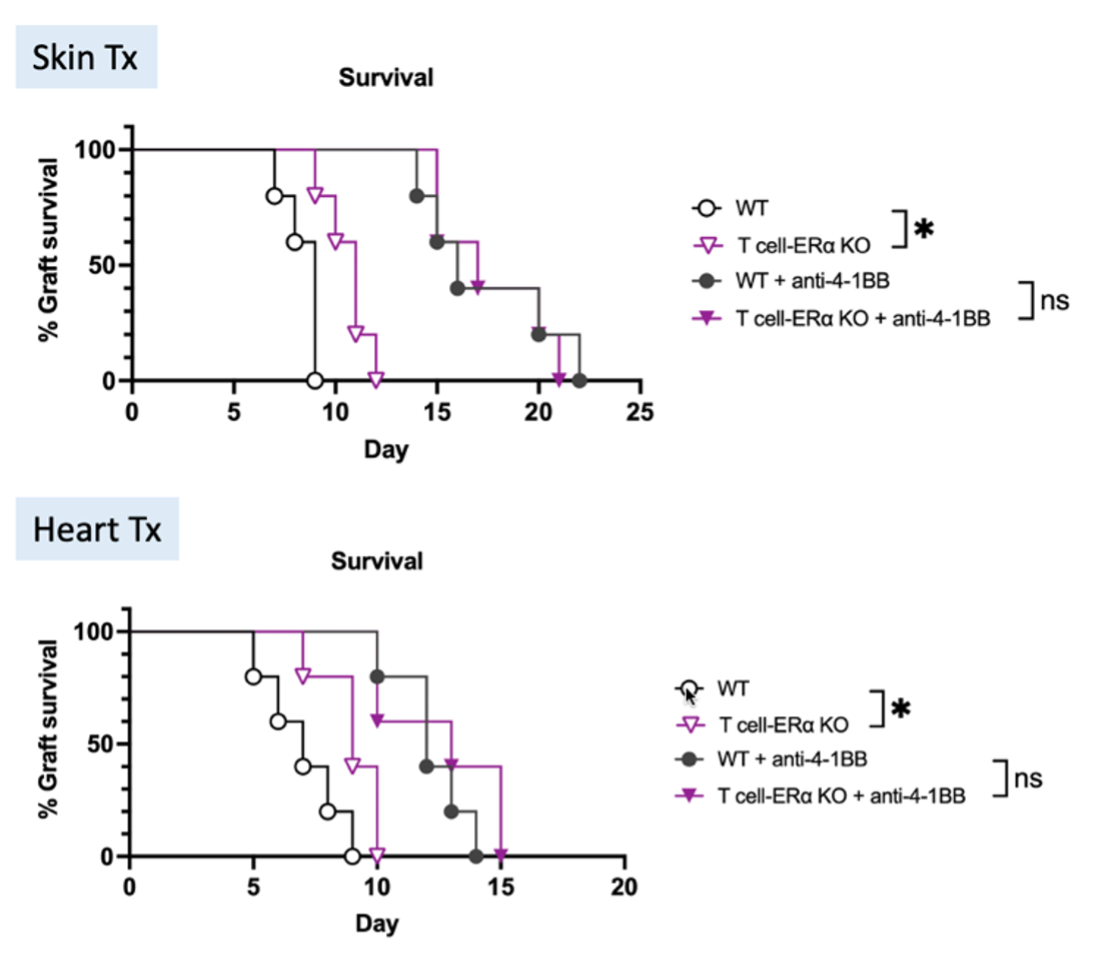
Conclusion: The ERα T-cell receptor is critical in promoting estrogen-mediated alloimmunity by enhancing 4-1BB co-stimulation (Figure 2).
{AbstractFigure.2}}
This work presents, for the first time to our knowledge, detailed mechanistic insights into sex hormone-driven immunological pathways with relevance in and beyond organ transplantation, providing a rationale for future therapeutic strategies targeting the ERα-4-1BB signaling axis.
S.G.T. receives grants from the National Institutes of Health (R01AG064165 and U01AI132898) and is supported by the Pablo and Almudena Legorreta Kidney Health Research Fund. Y.X. and F.M. are supported by the Women in Transplantation Research Fellowship Grant. K.N. and T.M. are supported by the Osaka Medical Foundation Scholarship.
[1] Biological sex
[2] Estrogen receptor
[3] 4-1BB
[4] alloimmune response
[5] costimulation
[6] acute rejection
[7] sex hormone
