Single cell RNA-sequencing delineates CD8+ tissue resident memory T cells maintaining rejection in liver transplantation
Xinqiang Li1, Shipeng Li2, Xin Zhou1, Feng Wang1, Qi Ling3, Jinzhen Cai1.
1Organ Transplantation Center, Affiliated Hospital of Qingdao University, Qingdao, People's Republic of China; 2Department of Hepatopancreaticobiliary Surgery, Henan Provincial People’s Hospital, Zhengzhou, People's Republic of China; 3Department of Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People's Republic of China
Understanding the immune mechanisms associated with liver transplantation (LT), particularly the involvement of tissue-resident memory T cells (TRMs), represents a significant challenge.
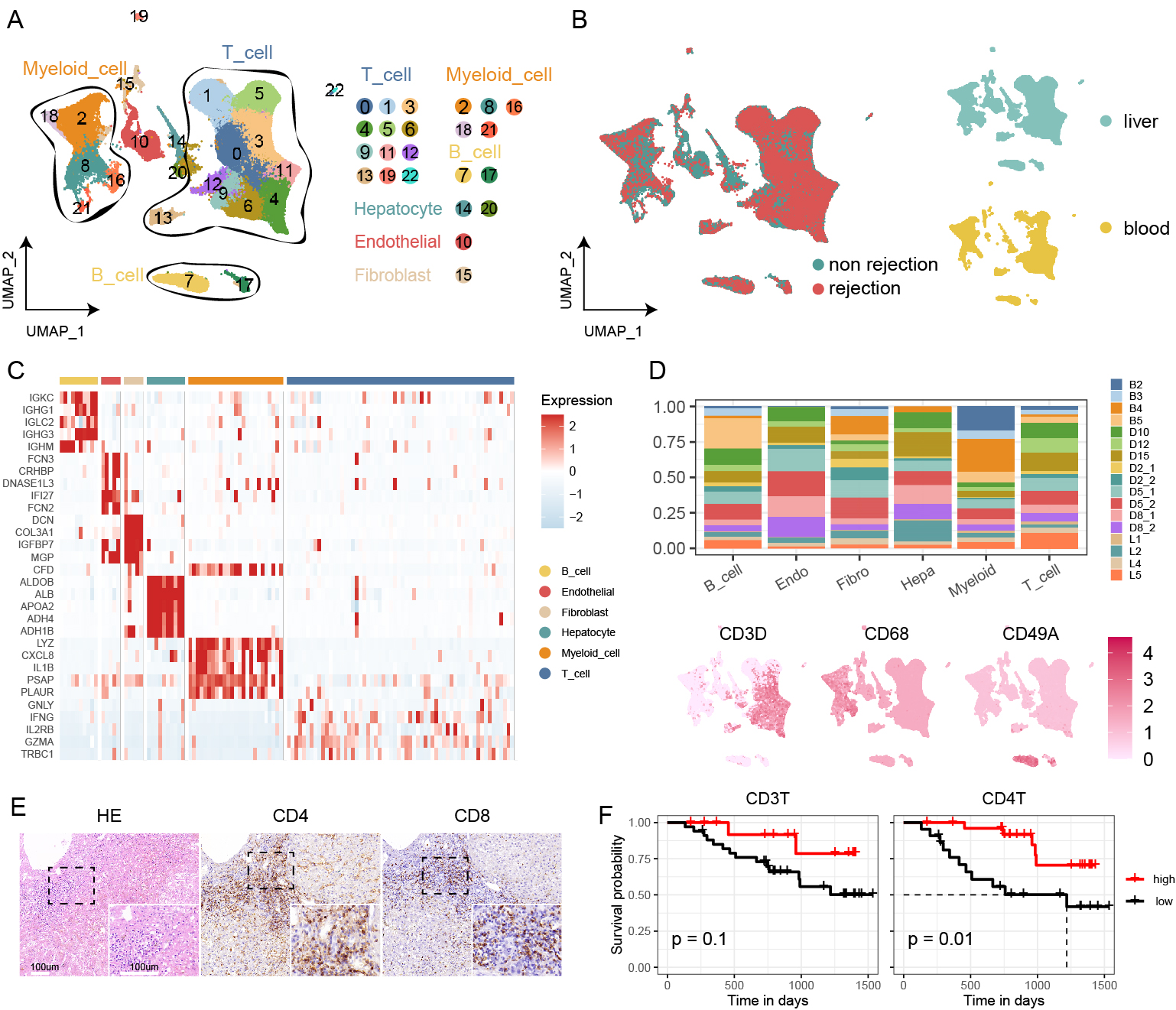
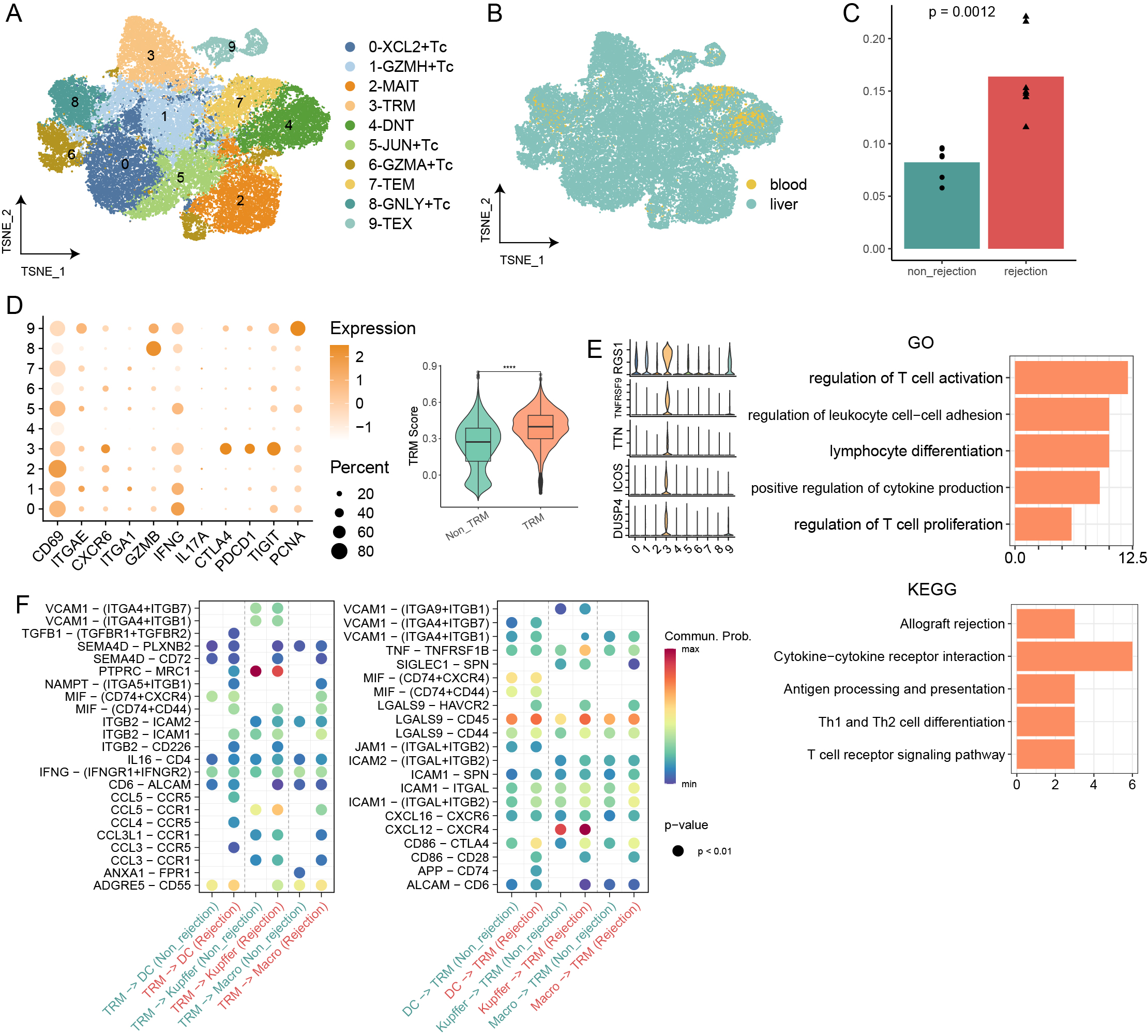
This study employs a multi-omics approach to analyse liver transplant samples from both human (n=17) and mouse (n=16), utilizing single-cell RNA sequencing, bulk RNA sequencing, and immunological techniques. Our findings reveal a comprehensive T cell-centric landscape in LT across human and mouse species, involving 235,116 cells. Notably, we found a substantial increase in CD8+ TRMs within rejected grafts compared to stable ones. The elevated presence of CD8+ TRMs is characterised by a distinct expression profile, featuring upregulation of tissue-residency markers (CD69, CXCR6, CD49A and CD103+/-,), immune checkpoints (PD1, CTLA4, and TIGIT), cytotoxic markers (GZMB and IFNG) and proliferative markers (PCNA and TOP2A) during rejection. Furthermore, there is a high expression of transcription factors such as EOMES and RUNX3. Functional assays and analyses of cellular communication underscore the active role of CD8+ TRMs in interacting with other tissue-resident cells, particularly Kupffer cells, especially during rejection episodes.
These insights into the distinctive activation and interaction patterns of CD8+ TRMs suggest their potential utility as biomarkers for graft rejection, paving the way for novel therapeutic strategies aimed at enhancing graft tolerance and improving overall transplant outcomes.
This work received support from the National Natural Science Foundation of China (No. 82370666), the Science Foundation of Shandong Province (No. ZR2022MH292) and the support from Science Foundation of Fujian Medical University Union Hospital (No. 2021XH025). The authors thank Dr. Ruixia Li (Harbin Medical University) and Dr. Haitao Huang (Zhejiang University) for the kindly assistance.
[1] liver transplantation
[2] tissue-resident memory T cells
[3] multi-omics
[4] graft rejection
[5] immune tolerance
