Lifei Liang, People's Republic of China has been granted the TTS Basic and Translational Mentee-Mentor Award
VISTA alleviates post-transplantation acute rejection by modulating ERK pathway and macrophage polarization
Lifei Liang1,2, Cuidi Xu1,2, Xiaohan Yu1,2, Cheng Yang1,2,3, Ruiming Rong1,2.
1Department of Urology, Zhongshan Hospital of Fudan University, Shanghai, People's Republic of China; 2Shanghai Key Laboratory of Organ Transplantation, Shanghai, People's Republic of China; 3Fudan Zhangjiang Institute of Fudan University, Shanghai, People's Republic of China
Introduction: Acute rejection is the main cause of graft failure, and more exploration is needed to improve graft survival after transplantation. V-domain Ig suppressor of T cell activation (VISTA) is a negative regulator expresses mainly on myeloid cells and naïve T cells that maintains T-cell quiescence. In this study, we demonstrated that vista could alleviate acute rejection of allograft by mediating the differentiation and polarization of macrophages.
Methods: Single cell sequencing was employed to identify the cellular source of VISTA. Abdominal mouse heart transplantation was performed, including isogeneic, allogeneic, WT and Vsir-/- groups. Samples were collected on day5, and the intensity of graft injury and rejection were evaluated by H&E staining and IF staining. Through joint Cut&Tag and RNA-sequence analyses, we identified H3K27ac as targeted enhancer and NF-κB as downstream pathway. Western-blot and RT-qPCR were applied in PMA-treated THP-1 to explore the mechanism of VISTA affect macrophages in vitro.
Results: Single cell analysis revealed that VSIR was dominantly expressed in monocytes and macrophages and was significantly changed in groups of macrophages after transplantation. Specific overexpression of VISTA in recipient macrophages prior to transplantation mitigated acute rejection, attenuated allograft injury and curbed immune cells infiltration. Besides, the survival curve showed longer survival of allograft with VISTA compared with allogeneic group. We further discovered that macrophages overexpressing VISTA exhibited a reduced proportion of inflammatory macrophages and an increased proportion of anti-inflammatory ones, compared to the control group. It was also validated in vitro. Then we found VISTA regulated ERK pathway by switching H3K27ac enhancer, providing a deeper insight into the mechanism by which VISTA influences macrophage polarization. By modulating the VISTA in recipient macrophages to ameliorate acute rejection, and altering factors associated with immune recognition, we hypothesized that the expression of VISTA in donor-resident macrophages played a pivotal role in the initiation of the rejection response post-transplantation. We performed abdominal heart transplantation with vsir-/- mice as donors and found reduced survival time of the transplanted heart and more severe tissue injury.
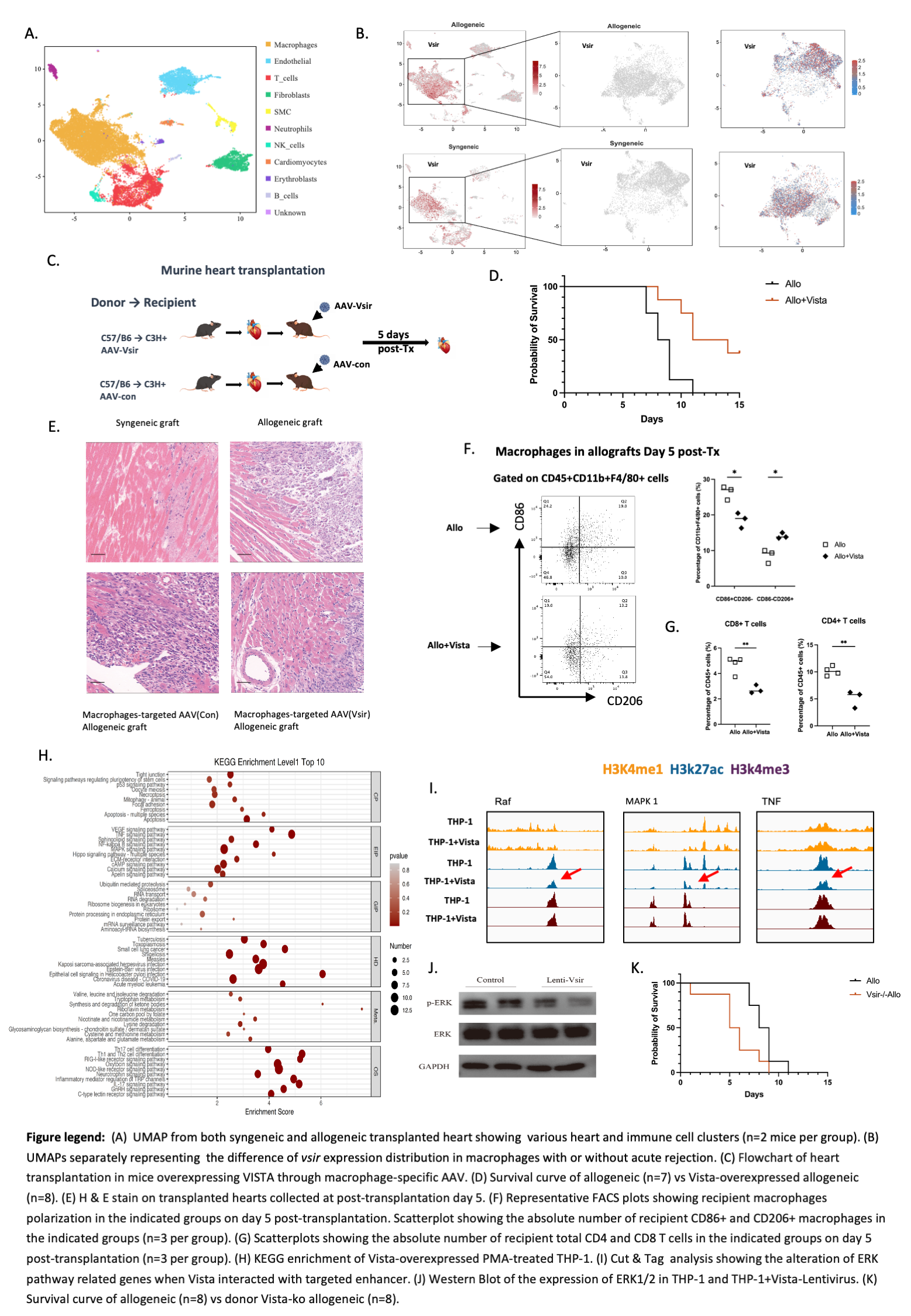
Conclusion: Vista could weaken the innate immune response of monocytes and macrophages to antigenic stimuli and affect the initiation of post-transplantation acute rejection, thereby altering the proliferation and polarization of inflammatory macrophages, inhibiting adaptive immunity and alleviating allograft acute rejection. Using VISTA before transplantation could be a potential therapy preventing allograft rejection.
