Macrophage nogo-B facilitates liver fibrosis
Lei Zhang1, Ming Ni1, Feng Cheng1, Long Zhang1, Yuan Liang1, Mu Liu1, Wenzhu Li1, Junda Li1, Yongquan Chi1, Jianhua Rao1, Ling Lu1.
1Hepatobiliary Center, The First Affiliated Hospital of Nanjing Medical University, Nanjing, People's Republic of China
Introduction: Liver fibrosis is characterized by sustained injury stress and chronic inflammation, repeated cell death and repair, all of which promote the progression of end-stage liver diseases (e.g. liver cirrhosis, carcinoma). As an endoplasmic reticulum-residential protein, Nogo-B possesses strong regulation abilities on macrophage function, but whether Nogo-B-decorated macrophage affects inflammation and progression is unclear during liver fibrosis. Herein, our aim is to elucidate the roles of Nogo-Bhigh macrophage during the liver fibrosis development.
Method: Expression and distribution of Nogo-B were analyzed with clinical specimens and animal models. Utilizing myeloid-specific Nogo-B knockout (Nogo-Bmko) mice, the mechanism and functionality of Nogo-Bhigh macrophage were investigated in three murine liver fibrosis models, which were induced separately by the bile duct ligation (BDL), the methionine- and choline-deficient (MCD) diet, and the carbon tetrachloride (CCl4) administration.
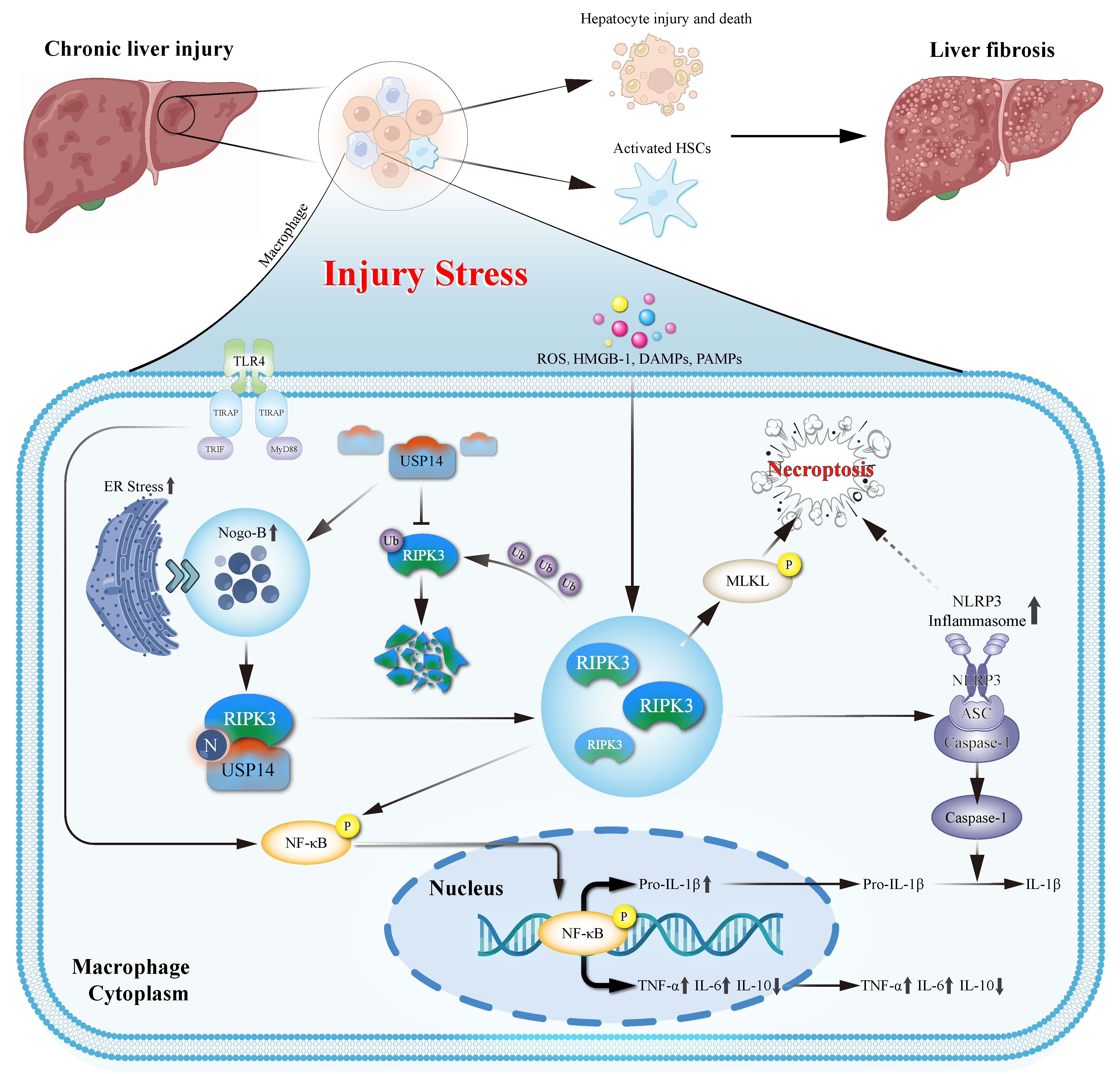
Results: Our study found predominant expression of Nogo-B in fibrotic liver macrophages and their positive correlation with fibrotic stages. Myeloid-specifific Nogo-B deficiency effectively alleviated liver inflammation, injury and fibrosis in three animal models. Importantly, Nogo-B deficiency inhibited NLRP3 inflammasome activation and necroptosis of macrophages in vivo and in vitro. Notably, RIPK3 was vital for Nogo-B driving NLRP3 inflammasome activation and necroptosis of macrophages. Additionally, adoptive transfer of macrophages indicated Nogo-B/RIPK3 axis promoted NLRP3 inflammasome activation and necroptosis, and accelerated liver fibrosis. Mechanistically, Nogo-B recruited USP14 restricted the ubiquitination degration of RIPK3 and enhanced RIPK3 stabilization.
Conclusions: Nogo-B facilitates liver fibrosis, by recruiting deubiquitination enzyme USP14, which increases stabilization of RIPK3 and promotes NLRP3 inflammasome activation and necroptosis in macrophages.
National Natural Science Foundation of China (81871259 and 81971495). Foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional Materials. The Priority Academic Program Development of Jiangsu Higher Education Institutions. The six talent peaks project in Jiangsu Province (2017-WSW-019).
[1] chronic liver disease
[2] macrophages
[3] hepatic fibrosis
[4] immunology in hepatology
