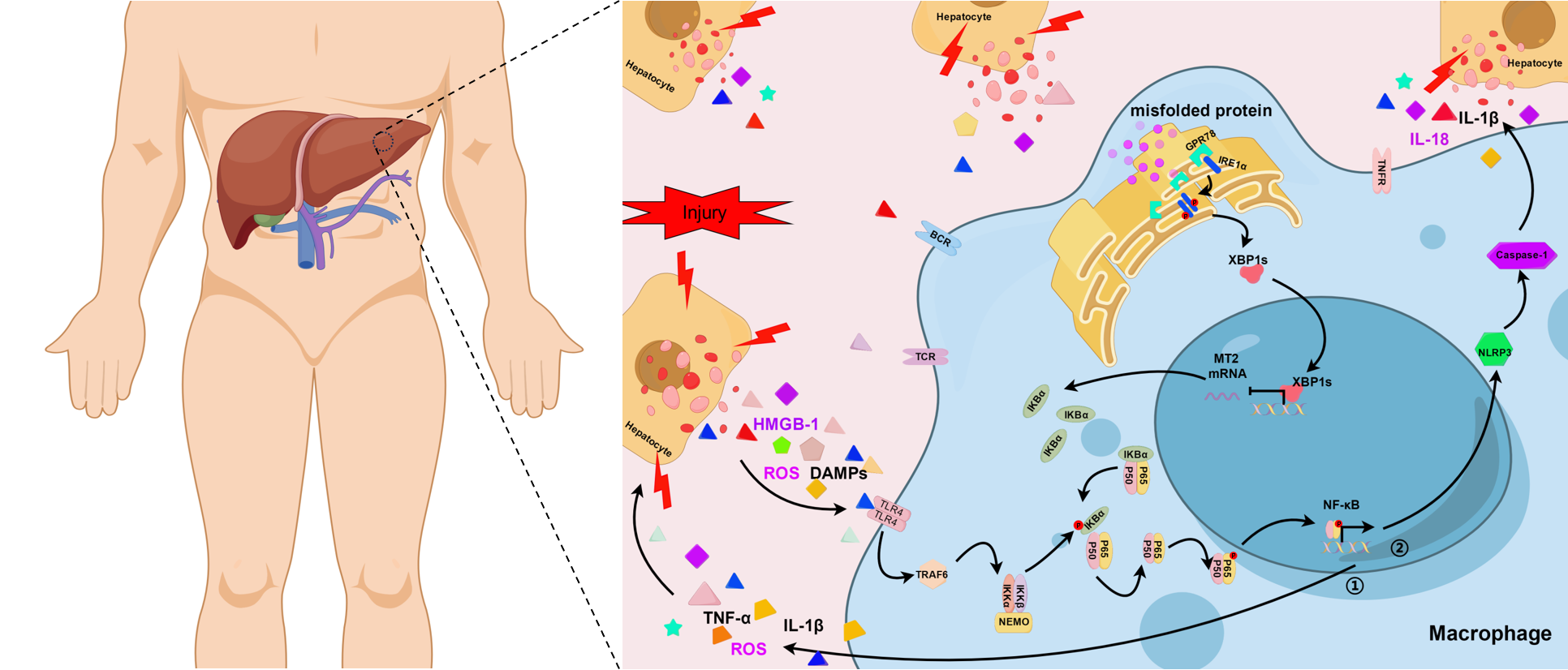
XBP1 facilitating NF-κB-p65 nuclear translocation promotes macrophage-originated sterile inflammation via regulating MT2 transcription in the ischemia/reperfusion liver
Jianhua Rao1, Zeng Wang1, Fei Yu1, Junda Li1, Zhengfeng Xuan1, Yongquan Chi1, Feng Zhang1, Liming Tang2, Feng Cheng1.
1Hepatobiliary Center, The First Affiliated Hospital with Nanjing Medical University, Nanjing, People's Republic of China; 2Center of Gastrointestinal Disease, The Affiliated Changzhou NO.2 People's Hospital of Nanjing Medical University, Changzhou, People's Republic of China
Introduction: XBP1, most conserved transcription factor of endoplasmic reticulum (ER) stress, plays important roles in physiological and pathological settings and has profound effects on disease progression and prognosis, so it’s necessary to investigate XBP1 in macrophage-originated sterile inflammation during liver ischemia/reperfusion injury (IRI).
Methods: Macrophage XBP1 expression and liver injury are analyzed in patients undergoing ischemia-related hepatectomy. A myeloid-specific XBP1-knockout (XBP1M-KO) strain is created for function and mechanism of XBP1 on macrophage-derived sterile inflammation in murine liver IRI with in-vitro parallel research. Macrophages co-cultured with hypoxia-treated hepatocytes are applied to investigate impact of XBP1 in vitro, with analysis of RNA sequencing and databases.
Results: Clinically, macrophage XBP1 expression significantly increases in ischemic liver tissues and positively correlates with liver injury after hepatectomy. Less hepatocellular damage is presented in XBP1M-KO mice than in XBP1-proficient (XBP1FL/FL) controls. In vitro, XBP1 deficiency inhibits sterile inflammation and migration in macrophages co-cultured with hypoxia-treated hepatocytes. Analysis of RNA sequencing and databases determines Metallothionein 2 (MT2) as XBP1 target gene, negatively regulated by binding with its promoter. XBP1 deficiency increases MT2 and IKBα expression, but inhibits NF-κB-p65 phosphorylation, markedly neutralizing XBP1M-KO-related benefits by promoting sterile inflammation during liver IRI.
Conclusion: XBP1 promotes macrophage-originated sterile inflammation, increases liver IRI by binding to MT2 promoter, and regulates MT2/NF-κB pathway, potentially therapeutic for clinical liver IRI.
The project was supported by the National Natural Science Foundation of China (8187125), The Six talent peaks project in Jiangsu Province (2017-WSW-019). The funding body had no role in the design of the study, data collection, analysis, interpretation, or writing the manuscript.
[1] Macrophages
[2] XBP1
[3] MT2
[4] NF-κB
[5] Ischemia/reperfusion injury
