Treatment with a specific inhibitor of the complement lectin pathway is protective against renal ischemia-reperfusion injury in mice
Anjan Bongoni1, Jennifer McRae1, Evelyn Salvaris1, Nella Fisicaro1, Bence Kiss2,3, Gábor Pál2,3, Péter Gál3,4, Peter Cowan1,5.
1Immunology Research Centre, St. Vincent’s Hospital, Melbourne, Fitzroy, Australia; 2Department of Biochemistry, Eötvös Loránd University, Budapest, Hungary; 3EvolVeritas Biotechnology Ltd., Budapest, Hungary; 4Institute of Molecular Life Sciences, HUN-REN, Research Centre for Natural Sciences, Budapest, Hungary; 5Department of Medicine, University of Melbourne, Melbourne, Australia
Introduction: Complement is a potent mediator of ischemia-reperfusion injury (IRI), which is an unavoidable event with detrimental effects after kidney transplantation. No effective clinical therapy to treat complement-mediated renal IRI and damage is currently available. Complement is activated in three ways, the classical, alternative and lectin pathways, and is tightly controlled by fluid-phase and membrane-bound regulators. Mannan-binding lectin–associated serine proteinase (MASP)-2 is essential for lectin pathway activation, which contributes to IRI and is a potential drug target. With using phage display, we developed a 100 picomolar human MASP-2 inhibitor protein, EVO24. For in vivo use, we fused a human IgG1-Fc to EVO24 resulting in the homodimer EVO24L, which selectively inhibits the lectin-pathway both in human and mouse serum.
Aims: To test EVO24L for the suppression of complement-mediated organ damage in a mouse model of renal IRI.
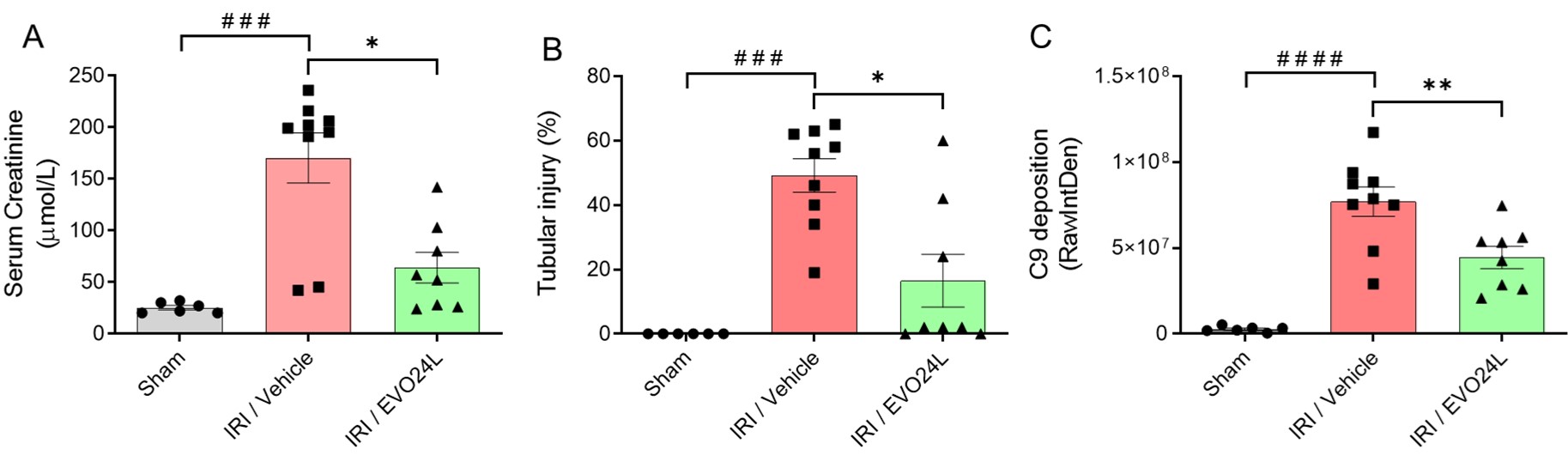
Methods: 10-12 week-old male C57BL/6 mice were subjected to right nephrectomy and left renal ischemia for 22 min (IRI), or nephrectomy only (Sham). Mice (n=8/group) were treated with 2 doses of EVO24L or vehicle control: 2 h pre-ischemia (600 mg/kg i.p.) and immediately post-reperfusion (300 mg/kg i.v.). Mice were euthanized 24 hr post-reperfusion, and serum and plasma samples were collected to assess renal function (serum creatinine, urea) and complement activation (C5a). Kidneys were harvested to analyse histopathology (tubular injury), complement C9 deposition and immune cell infiltration (neutrophils and macrophages).
Results: Compared to Sham, severe renal injury was induced following IR in vehicle-treated mice as indicated by significantly increased serum creatinine (170.2 ± 73.1 µM versus Sham: 25.2 ± 5.2 µM, p=0.0004, Fig 1A) and urea (344.2 ± 114.6 versus 60.1 ± 5.2 mg/dL, p<0.0001), plasma C5a (23.0 ± 8.3 versus 1.5 ± 1.0 ng/mL, p<0.0001), tubular injury (49.2 ± 15.7% versus Sham: 0.0 ± 0.5%, p<0.0001, Fig 1B), C9 deposition (7.7 ± 2.6 x 107 versus 2.8 ± 1.7 x 105 RawIntDen, p<0.0001, Fig 1C), and infiltration of neutrophils (49.0 ± 16.0 versus 0.0 ± 1.0/HPF, p<0.0001) and macrophages (50.0 ± 12.0 versus 0.0 ± 1.0 /HPF, p<0.0001). Compared to vehicle control, EVO24L treatment significantly protected against IR-induced damage, with significantly lowered serum creatinine (64.0 ± 42.1 µM, p=0.01 versus vehicle, Fig 1A) and urea (160.9 ± 144.7 mg/dL, p=0.02), plasma C5a (13.5 ± 5.5 ng/mL, p=0.015), tubular injury (16.5 ± 23.2%, p=0.01, Fig 1B), tissue deposition of C9 (4.5 ± 1.8 x 107 RawIntDen, p=0.008, Fig 1C), and infiltration of neutrophils (34.0 ± 9.0/HPF, p=0.04) and macrophages (33.0 ± 8.0/HPF, p=0.02).
Conclusion: Complement lectin pathway inhibition using EVO24L protected against kidney IRI. Blockade of lectin pathway activation by EVO24L is thus a promising therapeutic approach to reduce IRI and improve organ transplant function.
[1] Acute kidney injury
[2] Ischemia-reperfusion injury
[3] Lectin pathway
[4] Renal injury
[5] Novel therapeutics
[6] MASP-2 inhibitor
[7] Kidney transplantation
[8] Renal function
[9] Transplant kidney function
[10] Complement activation
