Efficacy of organ preservation at 22°C with extracellular-type preservation solutions in 2-hour warm ischemic kidney - Evaluation using a renal transplant model in MHC-inbred CLAWN miniature swine
Kazuhiro Takeuchi1, Akira Kondo1, Yuichi Ariyoshi1, Mitsuhiro Sekijima1, Akihiro Kawai2, Yurika Ichinari1, Akira Shimizu3, Mamoru Kusaka2, Masayoshi Okumi4, Hisashi Sahara1.
1Division of Experimental Large Animal Research, Center for Advanced Science Research and Promotion, Kagoshima University, Kagoshima, Japan; 2Department of Urology, Fujita Health University Okazaki Medical Center, Okazaki, Japan; 3Department of Analytic Human Pathology, Nippon Medical School, Tokyo, Japan; 4Department of Urology, Kyoto Prefectural University of Medicine, Kyoto, Japan
Introduction: In kidney transplantation with donors after cardiac death (DCD), warm ischemia (WI) of the graft is inevitable, which increases the risk of graft dysfunction. Currently, static cold preservation is the standard method of kidney preservation, however, kidneys from DCD donors are more susceptible to the effects of cold preservation. A previous study in small animal demonstrated the efficacy of storing DCD kidneys at 22°C with extracellular-type solutions (ETK®). Based on this finding, we evaluated the efficacy of storing WI kidneys at 22°C in MHC-inbred CLAWN miniature swine.
Methods: After 120 minutes of WI, the kidneys were preserved in ETK® for 60 minutes at either 4°C or 22°C. In experiment 1, the preserved kidneys were then subjected to ex vivo normothermic machine perfusion (NMP) with Ringer's solution containing red blood cells for 120 minutes at a mean arterial pressure of 85 mmHg. During this process, physiological and metabolic parameters were evaluated (n=3 in each group). In experiment 2, similar WI and preserved kidneys as in experiment 1 were transplanted into the MHC-matched recipients. Assessment of post-transplant renal function including serum creatinine (Cr) and renal biopsies over a 14-day (n=3 in each group). In addition, a WI kidney preserved in UW solution was transplanted into an MHC-matched recipient.
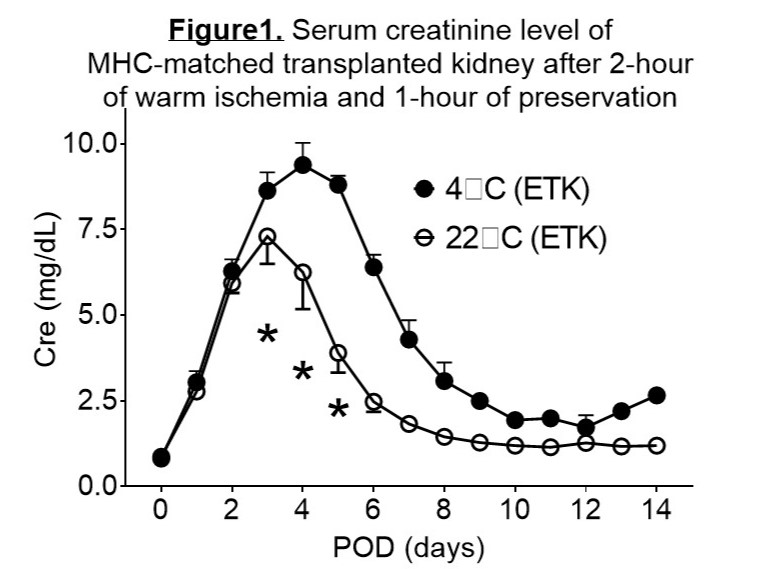
Results: In experiment 1, kidneys preserved at 22°C showed significantly better physiological and metabolic indices during ex vivo NMP compared to those preserved at 4°C. Specifically, mean renal blood flow, intrarenal resistance, total urine volume, and oxygen consumption were all significantly improved at 22°C (26.7 ± 4.7 ml/min vs. 10.0 ± 0.0 ml/min for blood flow; 3. 5 ± 0.5 mmHg/ml/min vs. 8.7 ± 0.1 mmHg/ml/min for resistance; 14.3 ± 5.3 ml vs. 5.4 ± 3.6 ml for urine volume; and 223.5 ± 38.6 ml/min/g vs. 92.5 ± 15.6 ml/min/g for oxygen consumption). In experiment 2, peak post-transplant serum Cr levels were significantly lower for kidneys stored at 22°C compared to those stored at 4°C (3.9 ± 0.6 mg/dl vs. 8.9 ± 0.3 mg/dl, p=0.0015; Figure 1). Kidney biopsies at postoperative day (POD) 4 showed extensive necrosis in the renal tubules of kidneys stored at 4°C, while damage was more localized in kidneys stored at 22°C. In addition, PCNA staining indicated a more rapid regeneration of the tubular epithelium in kidneys stored at 22°C. In contrast, the kidney stored in UW® exhibited severe renal dysfunction after transplantation and the recipient animal did not survive (peak Cr >15 mg/dl at POD7).
Conclusion: 2-hour WI kidneys from miniature swine showed that preservation at 22°C was more effective than at 4°C when an extracellular-type solution was used. To our knowledge, this is the first reported case demonstrating the efficacy of 22°C organ preservation in preclinical large animals.
[1] Organ Preservation
[2] Ischemia-reperfusion Injury
[3] Large Animal Model
[4] Subnormothermic Preservation
[5] Kidney Transplantation
[6] Porcine
