The combination of urine CXCL10 and donor-derived cell free DNA in the non-invasive diagnosis of antibody mediated and T cell mediated rejection in kidney transplantation
Daniel Fantus1,5, Silvia Casas3, Thierry Viard3, Narin Tangprasertchai3, Justin Bélair1, Chee Loong Saw4, Claude Daniel4, Julie Ho2, Héloise Cardinal1,5.
1Immunopathology, Centre de Recherche de CHUM, Montreal, QC, Canada; 2Internal Medicine, University of Manitoba, Winnipeg, MB, Canada; 3Research and Development Group, CareDx, Brisbane, CA, United States; 4HLA Laboratory, McGill University Health Center, Montreal, QC, Canada; 5Medicine, Centre Hospitalier de l'Université de Montréal (CHUM), Montreal, QC, Canada
Introduction: In kidney transplantation today, an allograft biopsy is required to diagnose rejection. Biopsies are invasive and difficult to use as a tool to monitor alloimmune activity over time. While serum creatinine is used clinically, it is neither sensitive nor specific for rejection. While there is increasing evidence that donor derived cell free DNA (dd-cfDNA) performs well as a biomarker of clinical antibody-mediated rejection (AMR), its ability to identify T cell mediated rejection (TCMR, including borderline rejection) remains unclear. In contrast, urine chemokines, such as CXCL10, are well-characterised biomarkers of tubulitis. Due to these complementary properties, we hypothesized that use of these 2 biomarkers together would improve the diagnosis of rejection phenotypes marked predominantly by tubulitis.
Method: A retrospective study was conducted whereby 126 kidney transplant biopsies were selected from the Centre Hospitalier de l’Université de Montréal transplant biobank. 120 of 126 biopsies had paired plasma and urine samples collected on the same day while the remaining biopsies had urine and plasma collected within 30 days. Banff 2019 criteria were followed to generate the following diagnostic categories: 20 cases of AMR (including suspicious AMR where 2 of 3 diagnostic criteria for AMR were met), 10 cases of low grade TCMR (Banff 1A or 1B), 7 cases of high grade TCMR (Banff 2B or greater) and 43 cases with normal histology (i,t,v,g and ptc scores=0). Banff borderline diagnoses were excluded. Urine CXCL10 was measured at the Chemokine laboratory, University of Manitoba using the Meso Scale V-Plex assay. Cell free DNA was extracted from EDTA plasma samples and percent of dd-cfDNA measured using the CareDx AlloSeq cfDNA assay (Brisbane, California). Cut-offs of 0.5% dd-cfDNA and 13 pg/ml urine CXCL10 (except for females less than 6 months post-transplant where we used a cut-off of 33 pg/ml) were selected for each assay, respectively.
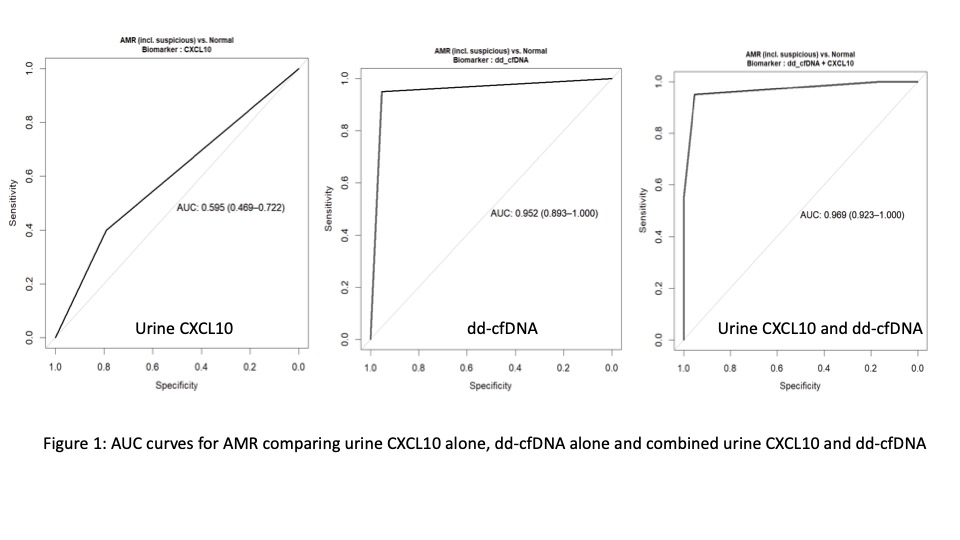
Results: The AUC for AMR (including suspicious AMR, compared to normal histology) was 0.952 (0.893-1000) using dd-cfDNA alone. In contrast, the AUC for urine CXCL10 alone for AMR was 0.595 (0.469-0.722) and increased to 0.969 (0.923-1.000) when combined with dd-cfDNA (p=1.71X10-8) (see Figure 1). When examining high grade TCMR, AUC for dd-cfDNA alone was 0.762 (0.562-0.963). In contrast, AUC for urine CXCL10 alone was 0.681 (0.474-0.888) and increased to 0.792 (0.585-1.000) when dd-cfDNA was added (p=0.16). For low grade TCMR, AUC was 0.577 (0.442-0.711) for dd-cfDNA alone. AUC for urine CXCL10 alone was 0.595 (0.424-0.767) and increased to 0.652 (0.473-0.832) (p=0.32) when combined with dd-cfDNA.
Conclusion: Urine CXCL10 is a weaker diagnostic biomarker of AMR compared to dd-cfDNA. In contrast, when evaluating TCMR, there was no clear advantage of one biomarker over the other, though their combination may improve diagnosis. These findings require external validation and prospective studies.
Fondation de CHUM.
[1] Biomarkers
[2] Kidney Transplantation
[3] Urine chemokines
[4] Donor-derived cell free DNA
[5] Non invasive diagnostics
