Differences in high and low dose belatacept conversion effects in kidney transplant patients
Hay Me Me1, Tyler Tinkham2, Sumi Nair1, Sami Alasfar1, Raymond Heilman1, Rebecca Corey2, Pooja Budhiraja1.
1Nephrology, Mayo Clinic, Phoenix, AZ, United States; 2Pharmacy, Mayo Clinic, Phoenix, AZ, United States
Background: A belatacept-based immunosuppression regimen, with mycophenolate and prednisone, is used to improve allograft function and avoid calcineurin inhibitor (CNI) toxicity. However, the optimal dosing strategy to balance rejection prevention and infection risk remains unclear.
Methods: This single-center retrospective study from Mayo Clinic Arizona included deceased donor and living donor transplant recipients from 2004 to 2022. High-dose belatacept (10mg/kg) is given if transplanted within one month, and the low-dose (5mg/kg) after one month for CNI intolerance or suboptimal allograft function with chronicity changes on biopsy findings. Tacrolimus was discontinued immediately in the high-dose conversion group but was overlapped and tapered down within four weeks in the low-dose conversion group. We compared the effect of high-dose and low-dose on the outcomes of allograft functions at six months, one year after the switch, and the occurrence of rejection and infection within one year.
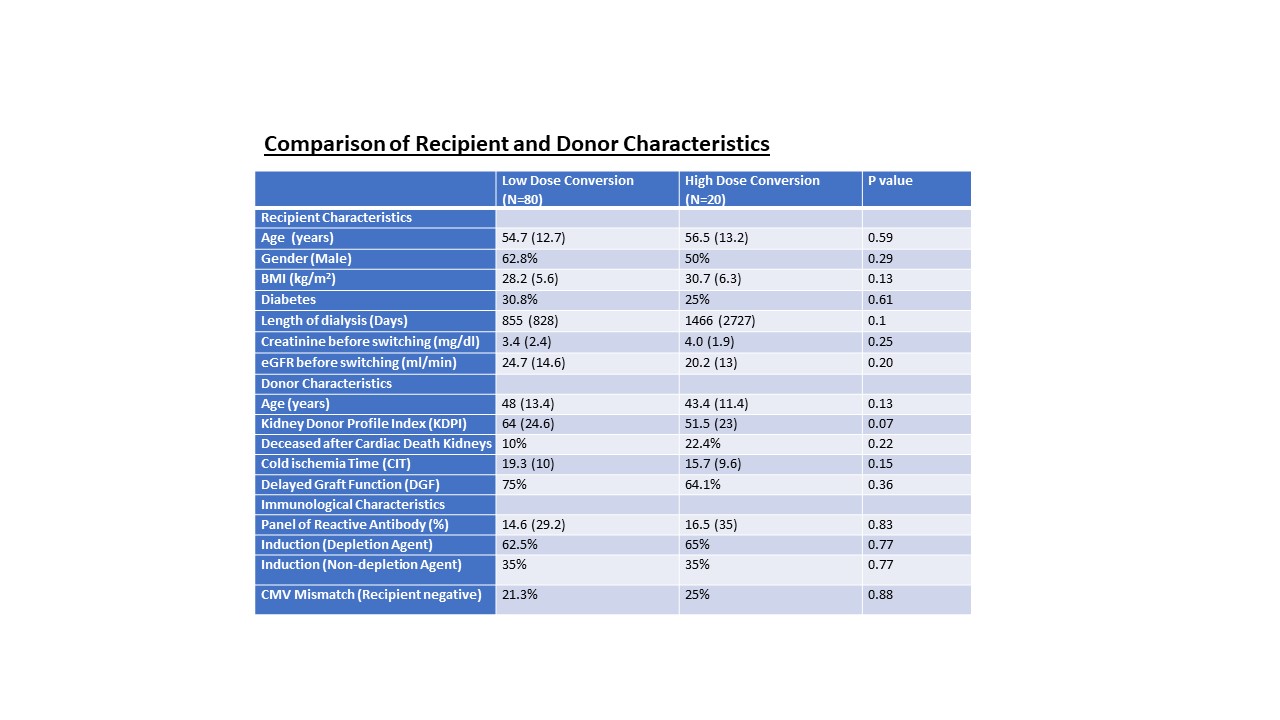
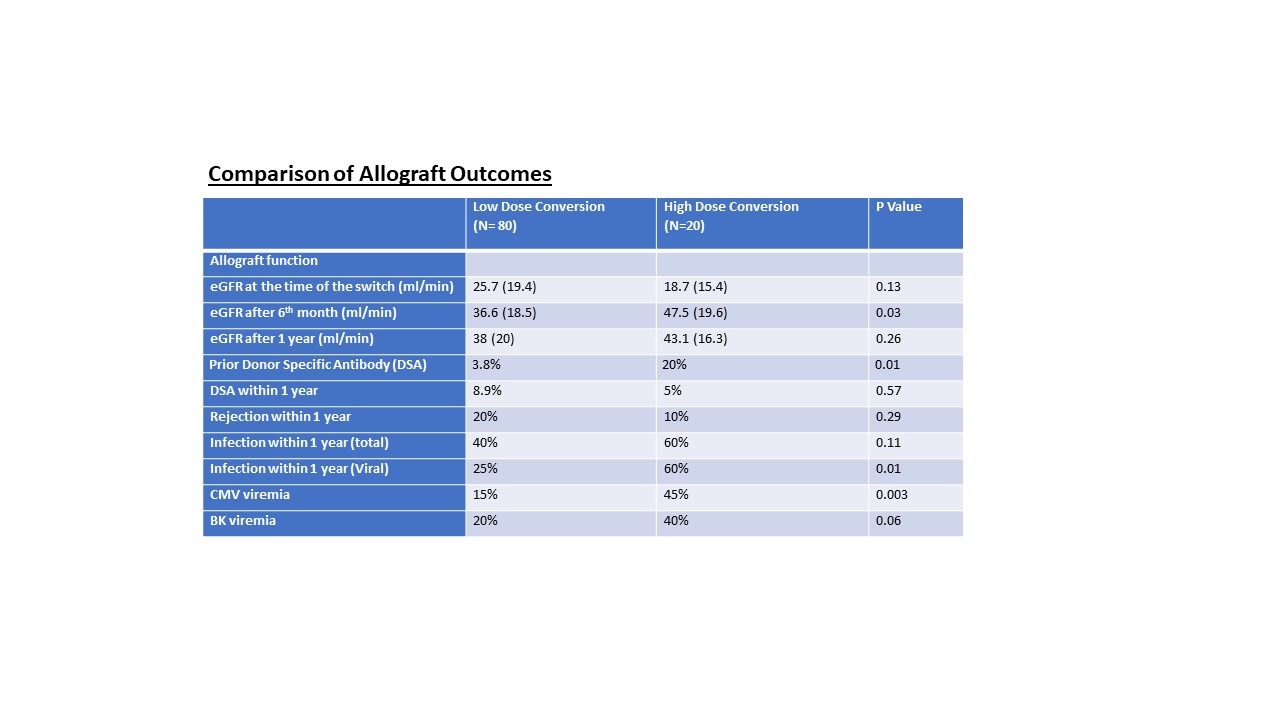
Results: 100 patients were converted to belatacept, 80 to low-dose, and 20 to high-dose protocol. No statistical differences were found between the groups except pre-formed donor-specific antibodies (DSA) higher in the high-dose group (Figure 1). The median days of belatacept initiation were 115.5 days (IQR 269.8) in the low-dose and 16.5 days (IQR 19.5) in the high-dose group (P=0.03). GFR significantly improved after six months and was steady at one year (Figure 2). There were more DSA in low-dose (8.9% vs. 5%) and more rejection episodes in low-dose (20% vs. 10%, P=0.29), mainly cellular rejection. Infection risk is statistically higher in the high-dose group (60% vs 25%), especially for viral infections, including CMV, BK, COVID, and EBV (Figure 2). One patient from each group had EBV viremia, and two patients from each group had COVID-19 infection. One graft loss was found in the high-dose and 11 graft loss were found in the low-dose group within one year.
Conclusion: Infection risk is higher in high-dose belatacept conversion, whereas rejection risk is higher in the low-dose belatacept conversion group, unrelated to induction therapy. Individualized dosing strategies should be considered to optimize outcomes while carefully monitoring infectious complications in belatacept patients.
[1] Immunosuppression
[2] Graft Rejection
[3] Infectious complications
[4] Allograft Survival
