Polarized HLA class I expression on renal tubules hinders the detection of donor-specific urinary extracellular vesicles
Liang Wu1,2, Martijn van Heugten1, Thierry van den Bosch3, Hans Duimel4, Carmen Lopez-Iglesias4, Dennis A. Hesselink1, Carla C. Baan1, Karin Boer1.
1Division of Nephrology and Transplantation, Department of Internal Medicine, Erasmus MC Transplant Institute, University Medical Center Rotterdam, Rotterdam, Netherlands; 2Department of Nephrology, The First Affiliated Hospital of Shaoyang University, Shaoyang, People's Republic of China; 3Department of Pathology, University Medical Center Rotterdam, Rotterdam, Netherlands; 4The Microscopy CORE Lab at the Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands
Introduction: Kidney transplantation is the optimal treatment for patients with end-stage kidney disease. Donor-specific urinary extracellular vesicles (uEVs) hold potential as biomarkers for assessing allograft status. We aimed to develop a method for identifying donor-specific uEVs based on human leukocyte antigen (HLA) mismatching with the kidney transplant recipients (KTRs).
Method: Urine and plasma were obtained from HLA-A2+ donors and HLA-A2- KTRs pre-transplant. CD9 (tetraspanin, EV marker) and HLA-A2 double-positive (CD9+ HLA-A2+) EVs were quantified using isolation-free imaging flow cytometry (IFCM). Healthy individuals’ urine was used to investigate CD9+ HLA-class-I+ uEV quantification using IFCM, time-resolved fluoroimmunoassay (TR-FIA), and immunogold staining cryo-electron microscopy (cryo-EM). Culture-derived CD9+ HLA-class-I+ EVs were spiked into the urine to investigate urine matrix effects on uEV HLA detection. Deceased donor kidneys and peritumoral kidney tissue were used for HLA class I detection with histochemistry.
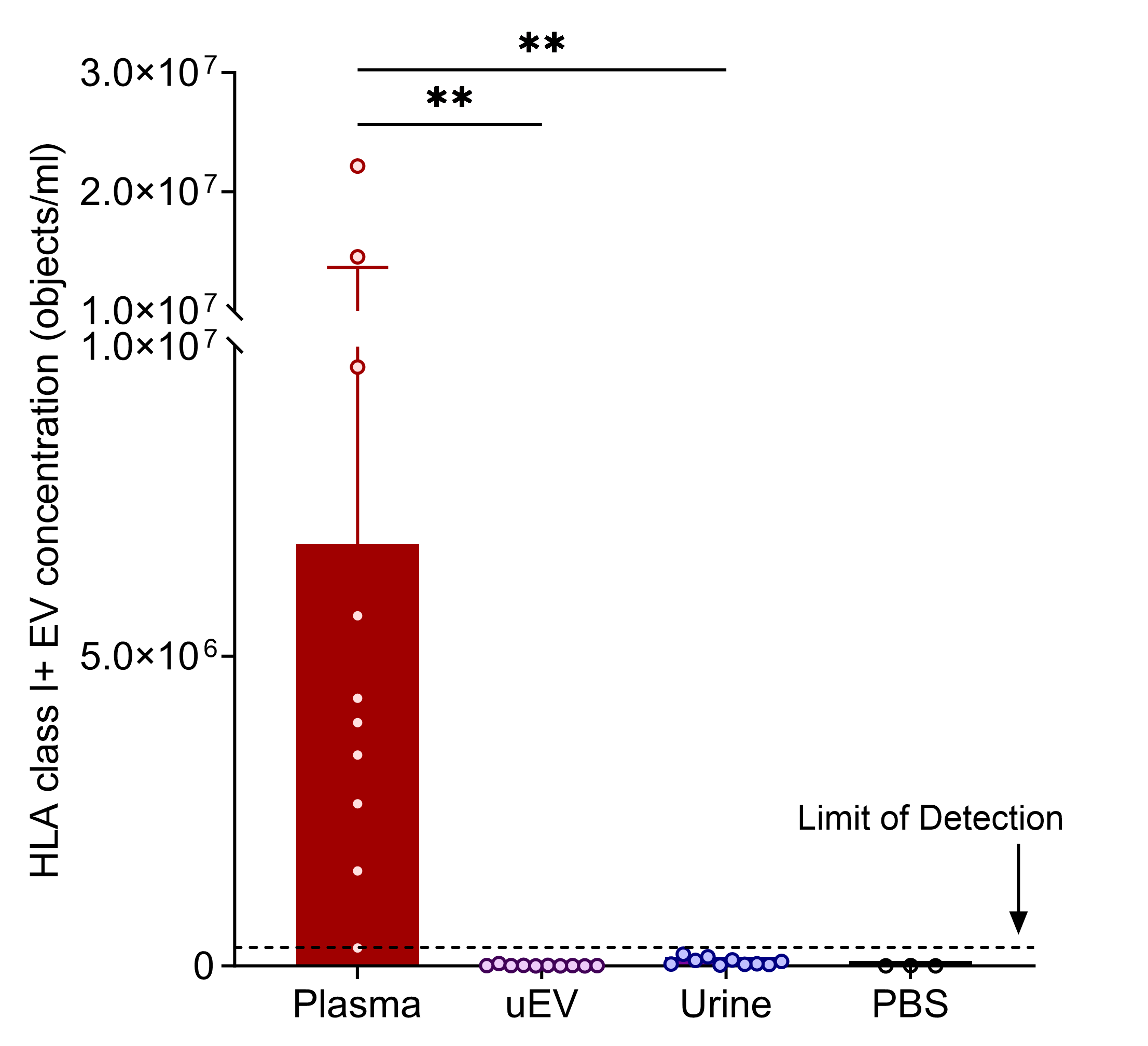
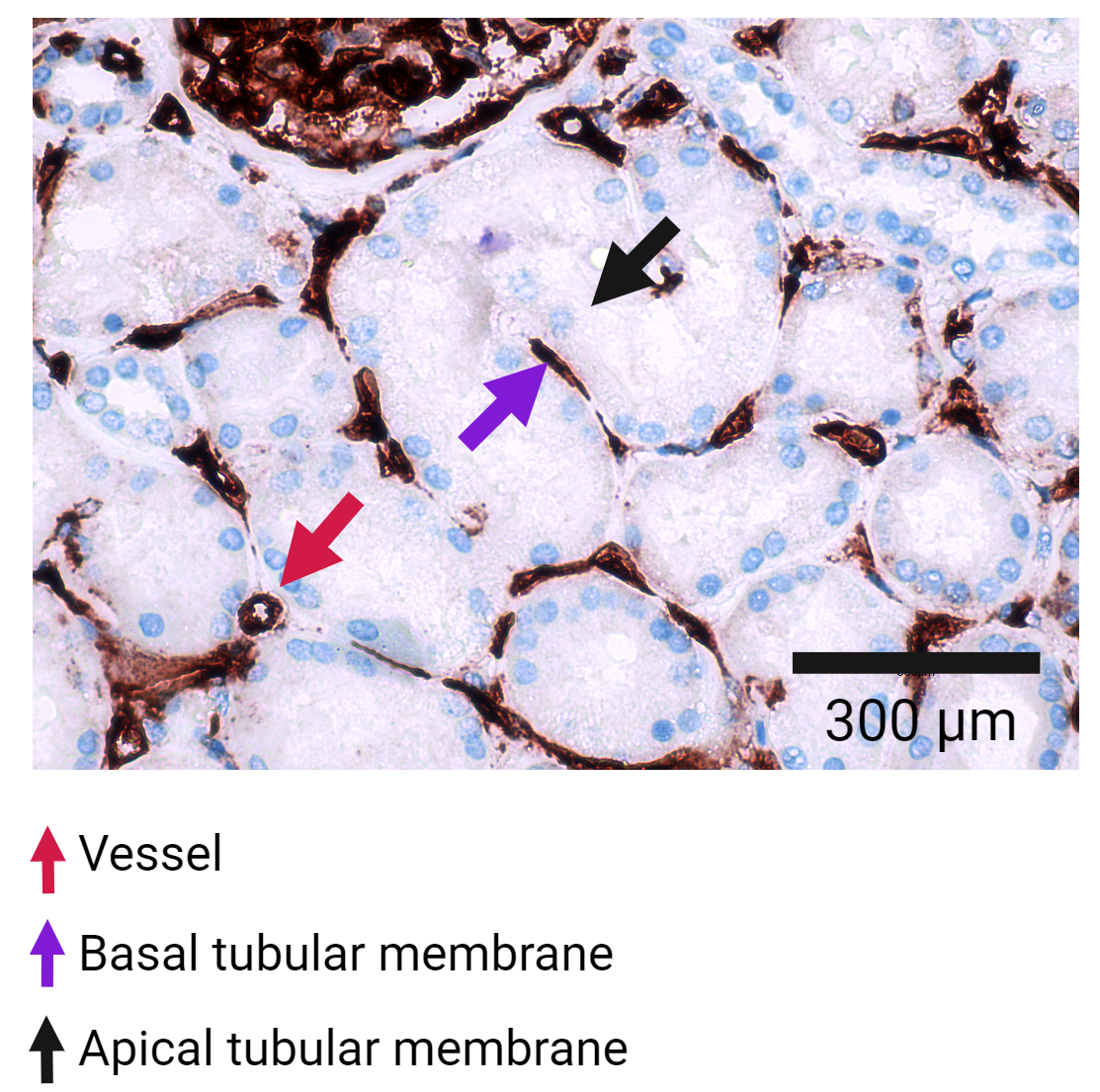
Results: The concentrations of CD9+ HLA-A2+ EVs in both donor and recipient urine approached the negative (detergent-treated) control levels for IFCM and were significantly lower than those observed in donor plasma. In parallel, universal HLA class I+ uEVs were similarly undetectable in the urine and uEV isolates compared with plasma, as verified by IFCM (Figure 1), TR-FIA, and cryogenic electron microscopy. Culture supernatant containing HLA class I+ vesicles from B, T, and human proximal tubule cells was spiked into the urine, and these EVs remained stable at 37°C for 8 hours. Immunohistochemistry revealed that HLA class I was predominantly expressed on the basolateral side of renal tubules, with limited expression on their urine/apical side (Figure 2).
Conclusion: The detection of donor-specific uEVs is hindered by the limited release of HLA class I+ EVs from the kidney into the urine, primarily due to the polarized HLA class I expression on renal tubules. Identifying donor-specific uEVs requires further advancements in recognizing transplant-specific uEVs and urine-associated markers.
We acknowledge Guido Jenster (Department of Urology, Erasmus Medical Center, Rotterdam, The Netherlands) for TR-FIA and the sponsorship from the China Scholarship Council (grant number 202008430154). All figures created on BioRender.com were provided with copyright licenses. All figures were created on BioRender.com with copyright licenses.
[1] Kidney Transplantation
[2] Donor-specific Biomarker
[3] HLA
[4] Extracellular Vesicles
[5] Human Urine
[6] Renal Tubule
