Appropriate risk classification and low-dose rituximab can help prevent antibody-associated rejection in ABO-compatible kidney transplants
Miruzato Fukuda1, Takayoshi Yokoyama1, Katsuyuki Miki2, Kiho Tanaka1, Yuki Nakamura2, Yasuo Ishii1.
1Center of Renal Surgery, Toranomon Hospital, Tokyo, Japan; 2Center of Renal Surgery, Toranomon Hospital Kajigaya, Kawasaki, Japan
Introduction: Rituximab desensitization therapy significantly improves the outcome of ABO-incompatible (ABOi) kidney transplantation (KT). Although ABO-compatible (ABOc) transplant cases are considered low-risk, cases of acute rejection are still observed, which strongly suggests the involvement of undetectable antibodies. A recent study suggested that administering 100 mg of rituximab could prevent antibody-associated rejection in patients who had undergone ABOi KT procedures and had low anti-A/B antibody titers, without causing complications. Based on this information, we hypothesized that proper risk classification and low-dose rituximab treatment might also effectively prevent rejection in patients who had undergone ABOc KTs. Since 2015, we have administered single low doses of rituximab (100 mg, given once) to some patients following ABOc KT procedures, to suppress rejection. This study compared the results of treating patients post-ABOc KT with rituximab vs without.
Methods: Among 135 ABO-c KT recipients between 2015 and 2022, 115— excluding those undergoing double filtration plasmapheresis (DFPP)—were divided into two groups: rituximab-free (n = 100) and low-dose rituximab (n = 15; Figure 1).
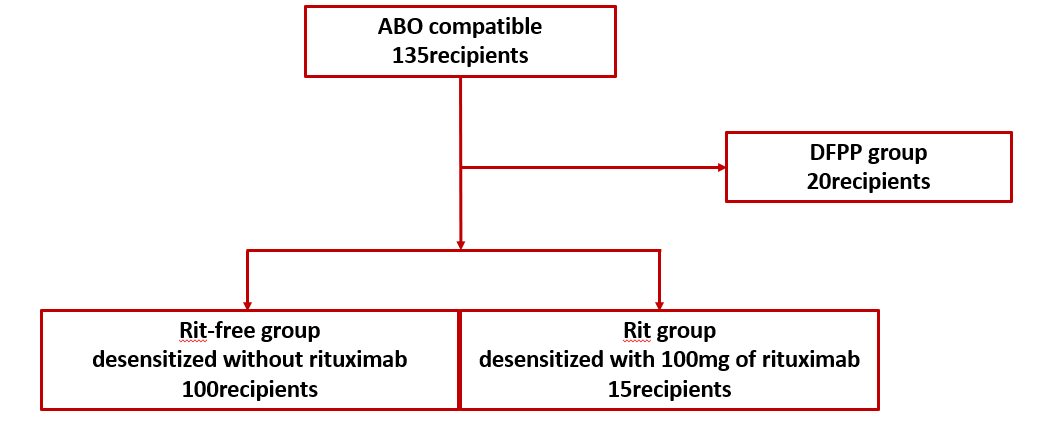
We evaluated the patients’ background, as well as the incidences of acute rejection, infection, and postoperative complications. Our primary focus was acute rejection within the first postoperative year, with other outcomes as secondary endpoints.
Results: The rituximab-free group had more KTs from related donors (66% [66/100]), whereas the low-dose rituximab group had more unrelated donors (73.3% [11/15]). There were no significant differences in postoperative renal function, as well as the incidences of complications or infection. The rituximab-treated group showed a significant decrease in the incidence of acute rejection compared to the untreated group (0.0% [0/15] vs. 8% [8/100], P = 0.223). In the untreated group, a higher percentage of rejections occurred in cases where the donor and recipient were not related than in cases where they were (6.1% [4/66] vs. 16.6% [4/24], P = 0.113). Even in low-risk populations, transplants from unrelated donors are considered high-risk. When the ABOc cases were examined regarding donor-specific antigen (DSA), a higher percentage of DSA(+) patients had prior histories of sensitization (61.1% [11/18]) and high mean fluorescence intensity (MFI) values.
Conclusion: We examined the use of low-dose rituximab following ABOc KT. In addition to genetic relationships, the risk factors for acute rejection include a prior history of sensitization and high MFI levels. As is shown in Figure 2, we propose a risk classification system.
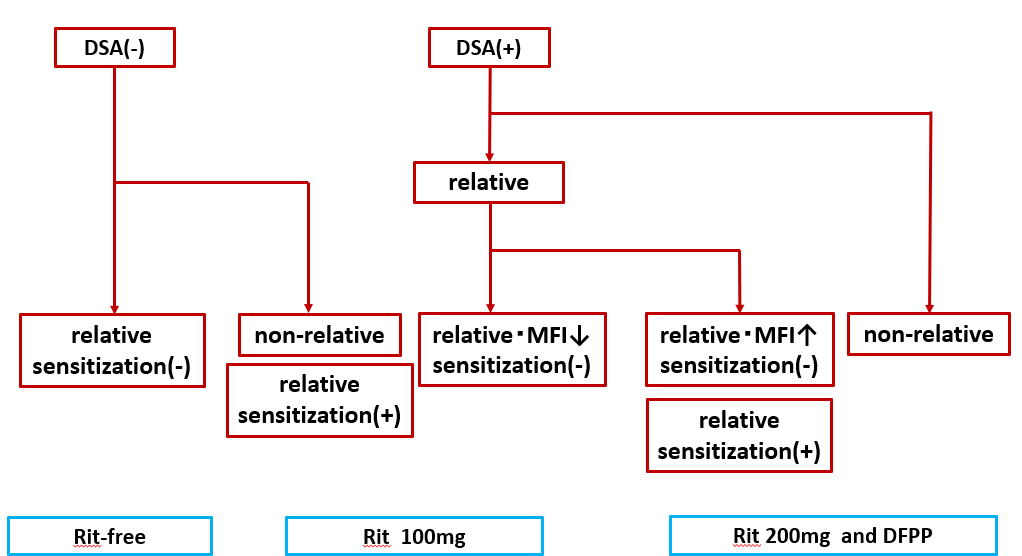
We propose that administering low-dose rituximab based on proper risk classification may decrease the occurrence of acute rejection, eliminate the need for DFPP, and minimize postoperative complications in patients undergoing ABOc KT procedures .
[1] ABO-compatible
[2] low-dose rituximab
[3] antibody-associated rejection
