Pilot study of clinical use of peripheral blood gene expression profile in the care of kidney transplant recipients between 2- and 5-years post-transplant
Sunil M Kurian1, James Fleming2, Bethany Barrick2, Allison Martin1, Chris Marsh1.
1Scripps Center for Organ and Cell Transplantation, La Jolla, CA, United States; 2Transplant Genomics, Inc., Framingham, MA, United States
Introduction: The purpose of this pilot study was to establish initial formative data of gene expression profile (GEP) used in the determination of patient treatment plans, including medication changes.
Methods: This was a single-center prospective pilot study to validate the clinical utility of serial GEP testing as an alternative to surveillance biopsies and optimize patient treatment plans. KTx who were between 12 and 60 months post-txp with stable srcr ( <2.3 mg/dL, <20% increase compared to previous 3 values) were eligible to be enrolled. Subjects enrolled in the study received TruGraf GEP tests every 3 months after enrollment for 2 years.
Results: Thirty subjects were enrolled between 4 and 12/2020. Two subjects only had 1 GEP test drawn (both resulted as “TX”) and were excluded. Median age was 64, 52% were male, and 59% were Caucasian (Table 1). Subjects were divided into cohorts based on TruGraf GEP results: “All TX” had only negative GEP results, “1 Not-TX” had one positive GEP result, and “>1 Not-TX” had 2 or 3 positive GEP tests. There were no differences in number of GEP tests between cohorts (7, 8.5, and 8 tests, respectively). The All TX cohort included subjects with the highest PRA and DSA. Out of the 4 rejections that occurred in the f/u, all occurred in All TX (1A, 2-1B, and 1 Chronic active rejection) and 75% occurred on the first or second visit). The 1B rejection on first visit subsequently developed BKVAN. All BKVAN cases (3) occurred in All TX or 1Not-TX cohorts. Contrary to previous observational early post-TX analyses, eGFR remained stable throughout the 2-year follow-up except for in the 1 subject that had 3 positive GEP tests (Figure 1). Interestingly, the combined IS trough (FK, SRL or FK+SRL) increased throughout follow-up for subjects in the >1Not-TX cohort, where subjects with either 0 or 1 positive GEP saw a trend towards reduced immunosuppressant trough values (Figure 2).
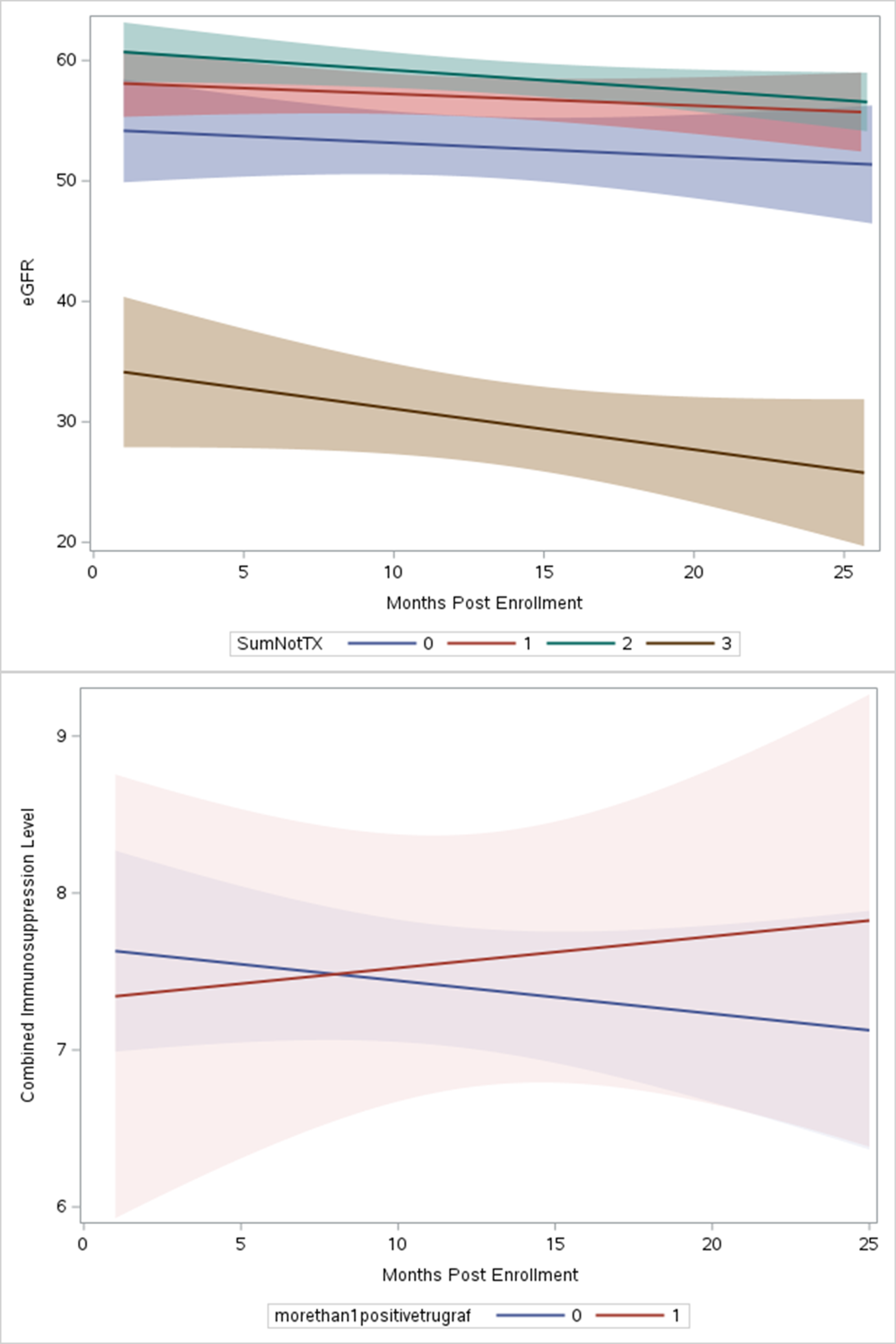
Conclusions: In this pilot study aimed at evaluating the clinical effectiveness of TruGraf GEP in real-time decision-making for patient treatment plans, individuals exhibiting multiple positive GEP tests were exposed to escalating doses of narrow therapeutic index immunosuppressants. Despite this, there were no AR episodes observed.They also experienced similar renal function compared to subjects with All TX, with the exception of 1 subject with 3 positive tests. The clinical outcomes were not consistent with previous findings in observational studies where the GEP result was not known. In those studies, positive GEP tests were associated with both increased rejection and worse renal function. This pilot study may indicate that GEP remains valuable for patients over a year post-txp, especially when immunosuppression isn't closely monitored as in immediate post-txp care and may prevent rejection and renal function decline. More studies with larger sample sizes are required to verify these early results.
[1] kidney transplant
[2] gene expression profile
[3] biomarkers
[4] rejection
[5] renal function
