A mate-kidney analysis examining the impact of Delayed Graft Function on kidney survival; implications for the clinician and clinical trial design
Ross Doyle1,2, Gal AvGay2, Ulrike Mayer2, John Gill2.
1Division of Nephrology, University of British Columbia, Vancouver, BC, Canada; 2Department of Nephrology, Mater Misericordiae University Hospital, Dublin, Ireland
Background: Delayed graft function (DGF) following kidney transplant is associated with inferior long-term outcomes. Trials examining interventions to mitigate DGF and its effects have been uniformly unsuccessful. Concordant DGF, where both recipients of kidney transplants from a single deceased donor, is poorly understood. The impact of concordant or discordant DGF on longer-term outcomes, specifically death censored graft loss (DCGL) has not been described systematically. We leveraged the power of a mate-kidney analysis to explore this relationship.
Methods: We performed a mate-kidney analysis, examining recipients of deceased donor kidney transplants between 2018-2019, using data extracted from the Scientific Registry of Transplant Recipients (SRTR). We identified rates of DGF, concordance and discordance in DGF. We devised competing risks models for graft failure, with death as a competing risk, to explore the impact of concordant/discordant DGF on DCGL.
Results: Of 11,547 deceased donors between 2018-2019 where both kidneys were transplanted into individual, first, kidney-only recipients there were 6,947 cases of DGF (30.1%); 1,778 (15.44%) donors where concordant DGF occurred, a rate that was higher than expected by chance alone, and which was higher for DCD compared to DBD donors. Discordant DGF occurred in 3,391 donors (29.3%), where one recipient developed DGF and the mate did not.
Among donors with discordant DGF, the cumulative incidence of DCGL in the mate who did not develop DGF was no different than that seen in recipients of kidneys from a donor where neither recipient developed DGF (HR 1.02, CI 0.86-1.20). This is most clearly seen among donors with KDPI 21-85%, and particularly in DBD donors, as seen in the middle panels in Figure 1.
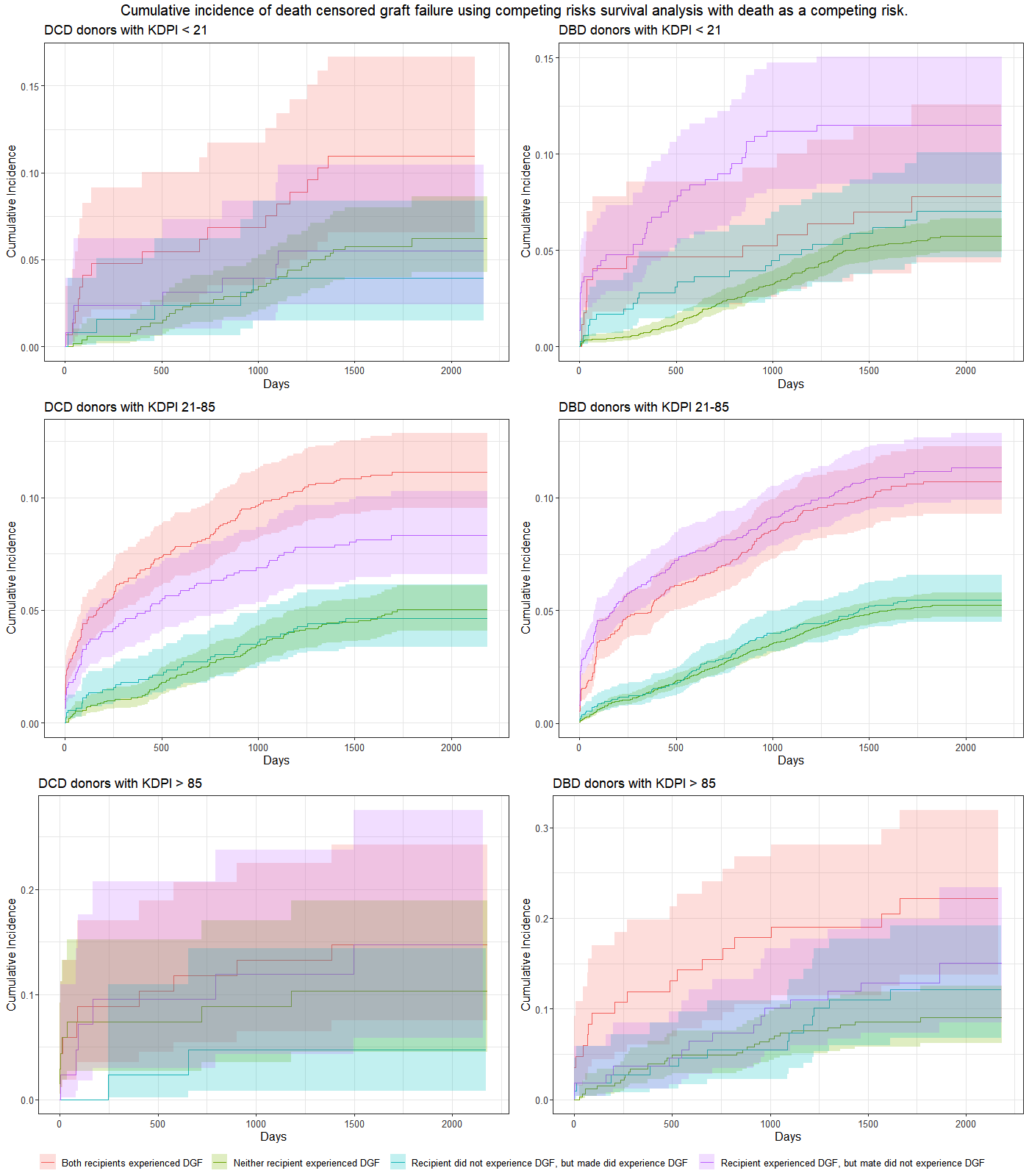
Conclusions: DGF impacts kidney survival, but among recipients of deceased donor transplants where there was discordant DGF, the kidney survival in the mate who did not develop DGF was not different to recipients of transplants from donors where neither recipient developed DGF.
For recipients of deceased donor transplants who themselves do not develop DGF, understanding the fate of the mate does not provide prognostic information, especially in the case of DBD donors.
In recipients of transplants from donors with KDPI 21-85%, development of DGF appears to be a major factor through which the increased risk of DCGL is mediated. Clinical trials examining interventions to attenuate DGF should not focus on the highest risk groups (i.e. KDPI ≥ 85%) alone, but should ensure adequate representation from donors with KDPI 20-85% also.
[1] Delayed Graft Function
[2] Deceased Donor Kidney Transplantation
[3] Graft survival
[4] Concordant DGF
[5] Clinical Trial Design
