Friederike Martin, United States has been granted the TTS Basic and Translational Mentee-Mentor Award

Re-assessing the determination of sensitization status in kidney transplant candidates: unveiling persistent anti-hla antibodies in waitlisted patients
Friederike Martin1,2, Elizabeth Zicari1, Merih Gizlenci1, Yao Xiao1, Keita Nakamori1,3, Yuko Sato1, Hao Zhou1, Edgar L. Milford4, Stefan G. Tullius1, Melissa Y. Yeung4.
1Division of Transplant Surgery, Department of Surgery, Brigham and Women’s Hospital, Harvard Medical, Boston, MA, United States; 2Department of Surgery, Campus Charité Mitte | Campus Virchow-Klinikum, Charité - Universitätsmedizin, Berlin, Germany; 3Department of Urology, Osaka Medical and Pharmaceutical University, , Osaka, Japan; 4Department of Medicine, Renal Division, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Introduction: Transplantation centers routinely report sets of unacceptable antigens for patients waitlisted for kidney transplantation to identify and exclude human leucocyte antigen (HLA) incompatible (“unacceptable”) donors for patients. Unacceptable antigen sets are further used by the United Network of Organ Sharing (UNOS) to generate the calculated panel reactive antibodies (cPRA) and centers can decide whether to list patients with their current or their peak cPRA. Here, we delineated which set of unacceptable antigens most accurately reflects the sensitization level of patients undergoing multiple screenings while waiting for a transplant.
Method: We conducted an analysis of 10,000,000 reports of unacceptable antigens from the UNOS Standard Transplant Analysis and Research (STAR) file (2023), including data from 153,226 kidney transplant candidates (10/1999 – 06/2023). Data analysis was performed using JMP®, Version 16.2.0, SAS Institute Inc., Cary, NC.
Results: The majority (54.13%) of all analyzed patients never developed antibodies against HLA class I or class II antigens. Among sensitized patients (47.9%, n=70,287), the vast majority (83.6%, n=58,792) made antibodies against HLA class I (A, B, C), while 55.3% (n=38,883) showed reactivity against class II (DRB1, DRB345, DQ). Only 20.5% (n=14,429) of sensitized patients never changed the number of reported antibodies, whereas 79.5% (n=55,858) presented with ≥ one change of the reported antibody count.
When analyzing the unacceptable HLA-A locus antibodies in a random subset of 2,432 waitlisted patients, the list of unacceptable antigens remained the same over time for the substantial majority (97.5%) in consecutive reports. The analysis of consecutive reports for individual patients supported that observation, demonstrating the significance of re-appearing antibody specificities in a particular patient once identified (Figure 1). In contrast, only 2.5% of consecutive reports revealed a change in the set of unacceptable antigens.
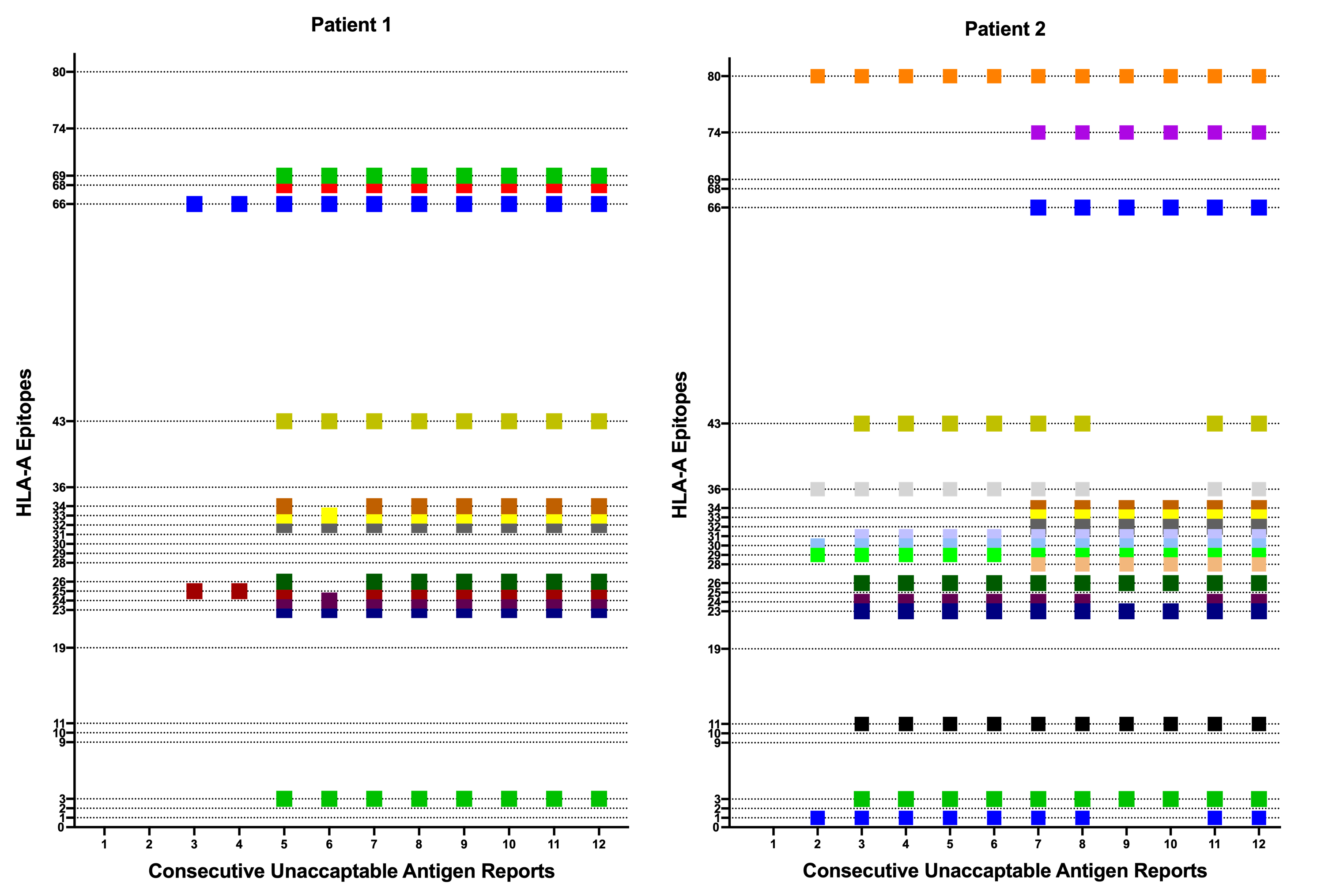
Conclusion: Our findings suggest that neither peak nor current cPRA fully display the extent of sensitization in candidates waiting for renal transplantation. The cumulative sum of reported unacceptable antigens over time, in contrast, provides a more accurate reflection of a patient's sensitization status. Implementing this change in the reporting structure is expected to improve organ availability and transplant outcomes for sensitized patients.
[1] Sensitization
[2] Unacceptable Antigens
[3] Calculated Panel Reactive Antibodies (cPRA)
[4] HLA Antibodies
