
T cell-derived IL17 orchestrating macrophages infiltration in chronic renal allograft rejection
Yanxu Chen1, Qiang Zhang1, Longshan Liu1, Changxi Wang1.
1Organ Transplant Center, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, People's Republic of China
Background: Chronic rejection, driven by intricate interactions among diverse immune cell populations,represents a primary factor contributing to kidney transplant failure. Understanding the mechanisms governing the evolution of chronic rejection offers a promising avenue for timely intervention aimed at prevent allograft failure.
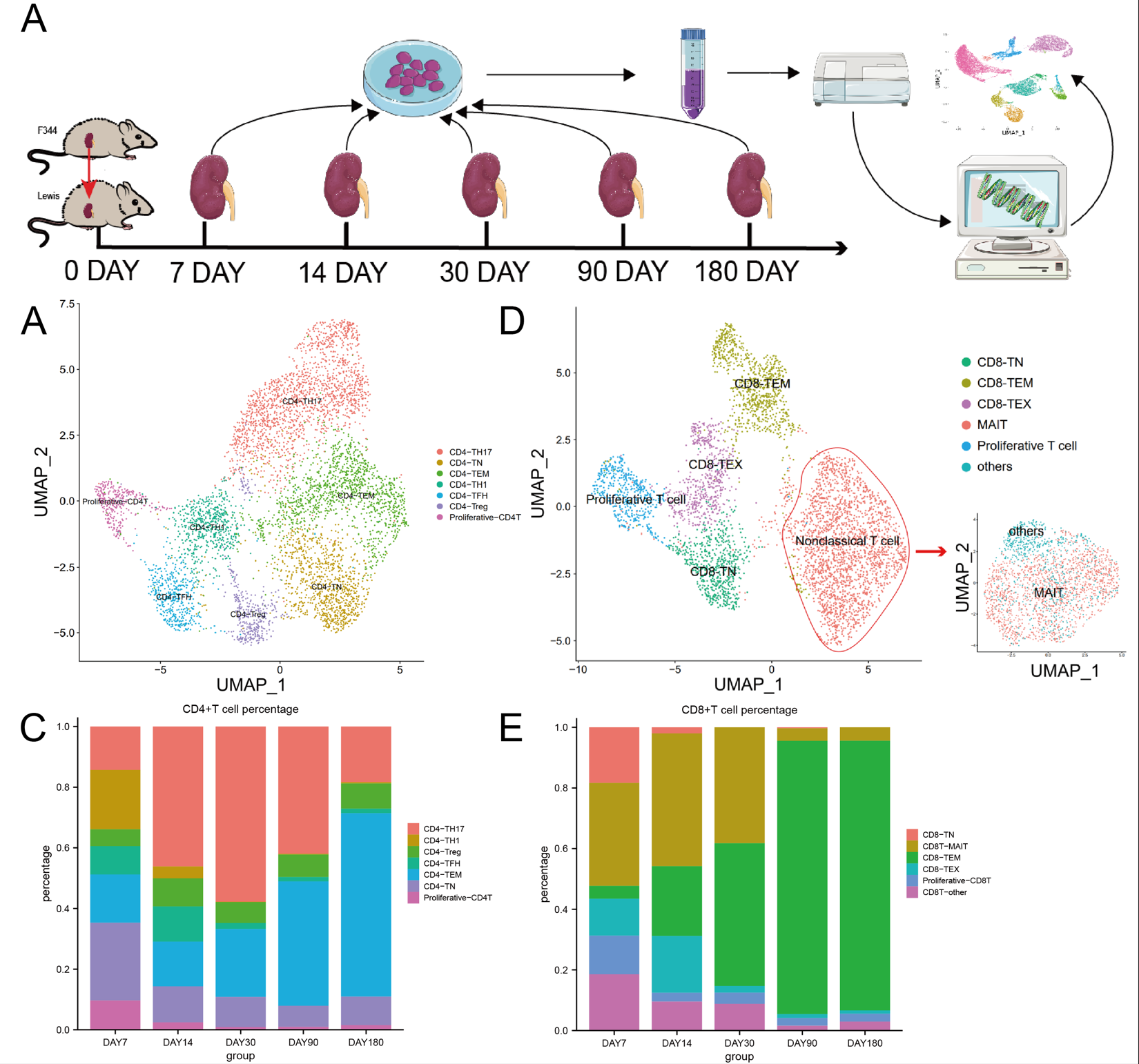
Methods: A chronic rejection model of kidney transplantation was established in rats (F344 to Lewis strain).Renal allografts were harvested for single-cell transcriptomic sequencing on days 7,14,30,90,and 180 post-operation. Monocle2 was used to delineate the evolutionary trajectory of Th17 subgroups, and Cellchat was utilized to investigate cellular communication networks. Immunofluorescence was performed to confirm changes in TH17 and MAIT cell infiltration.
Results: 30898 cells were obtained and classified into 11 clusters. The overall signal variations in the transplanted kidney during the early and late stages primarily manifested as the gradual decrease in the proportion and downregulated function of T cells. Further examination of T cells revealed that the TH17 subset within CD4+ T cells exhibited the most significant functional fluctuations, initially increasing in number and subsequently decreasing. Pseudotime analysis suggested a potential evolutionary link between TH17 and TH1 cells, with late-stage TH17 cells being the sole expressers of IL17a. Analysis of CD8+ T cells revealed a considerable proportion of MAIT cells, predominantly present during early rejection and primarily associated with IL17-related functions. Immunohistochemistry confirmed the sustained presence of IL17a during the progression of chronic rejection. Furthermore, an in-depth investigation into the evolving interaction signals between T cell subgroups and other cell types within the allograft microenvironment revealed persistent communication signals with myeloid cells. Macrophages, as the principal subgroup of infiltrating myeloid cell, also exhibited notable changes in both quantity and function throughout the rejection process, with IL17Ra+M1 demonstrating a consistent upward trend. IL17a played a crucial role in maintaining their function, with MAIT cells providing support beyond IL17a, including signals such as csf1 and IL34.
Conclusions: This study highlights the central role of T cells,particularly IL17-producing MAIT and Th17 cells, in the progression renal allograft chronic rejection. IL17,primarily generated by MAIT cells in early rejection and later by Th17 cells,promotes the continuous advancement of chronic rejection by directly regulating the mesenchymal transition of renal tubular epithelial cells and influencing the phenotype of macrophages.These findings offer valuable insights for the development of targeted IL17-based treatments for chronic rejection.
NSFC 82170770.
[1] chronic rejection
[2] IL17
[3] MAIT
[4] Macrophage
