
Association of blood dd-cfDNA levels with Banff scores and histopathological lesions in kidney allograft biopsies: results from an observational study
Aylin Akifova1, Klemens Budde1, Mira Choi1, Michael Oellerich2, Nils Lachmann3, Julia Beck4, Kirsten Bornemann-Kolatzki4, Ekkehard Schütz4, Bilgin Osmanodja1.
1Department of Nephrology and Intensive Care, Charité - Universitätsmedizin Berlin, Berlin, Germany; 2Department of Clinical Pharmacology, University Medical Center Göttingen, Göttingen, Germany; 3Centre for Tumor Medicine, Histocompatibility & Immunogenetics Laboratory, Charité - Universitätsmedizin Berlin, Berlin, Germany; 4Chronix Biomedical GmbH, Göttingen, Germany
Introduction: Donor-derived cell-free DNA (dd-cfDNA) is a novel biomarker of kidney allograft injury. While primarily studied to discriminate rejection from no rejection, knowledge about its dynamics in other graft pathologies merits further investigation. Recent research highlighted its ability to discriminate antibody-mediated rejection (ABMR) better than T-cell-mediated rejection (TCMR) and borderline changes but also suggested that its release is not limited to alloimmune-mediated injury. Therefore, we aimed to assess the association between dd-cfDNA and diverse histopathological patterns as well as its correlation with Banff scores in kidney allograft biopsies.
Methods: For this analysis, we identified all dd-cfDNA-paired biopsies from a prospective observational study of kidney transplant recipients (KTR) undergoing an indication biopsy due to graft dysfunction, persistence of anti-HLA donor-specific antibodies (DSA), or proteinuria. Dd-cfDNA was collected at biopsy and measured using droplet-digital PCR. Both relative and absolute quantification of dd-cfDNA, with cutoffs of 0.5% and 50cp/ml, respectively, were available for clinical interpretation. Biopsy specimens were examined by an experienced nephropathologist blinded to the dd-cfDNA results. Final diagnoses were assigned according to the Banff 2019 classification.
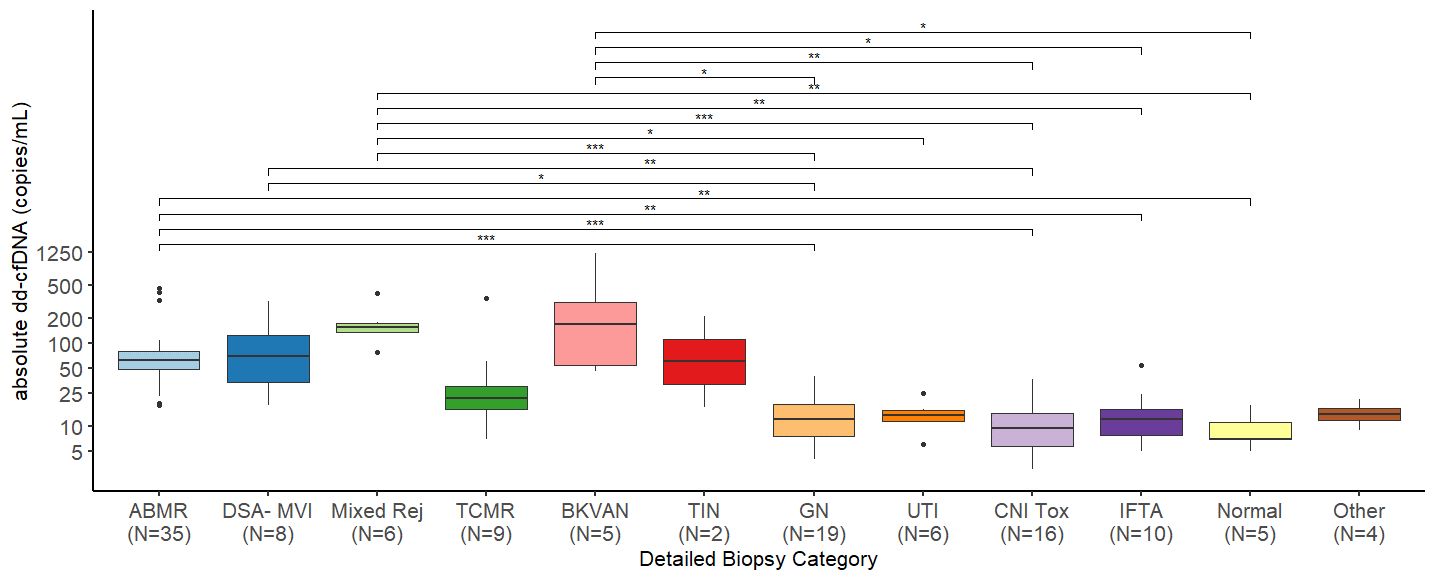
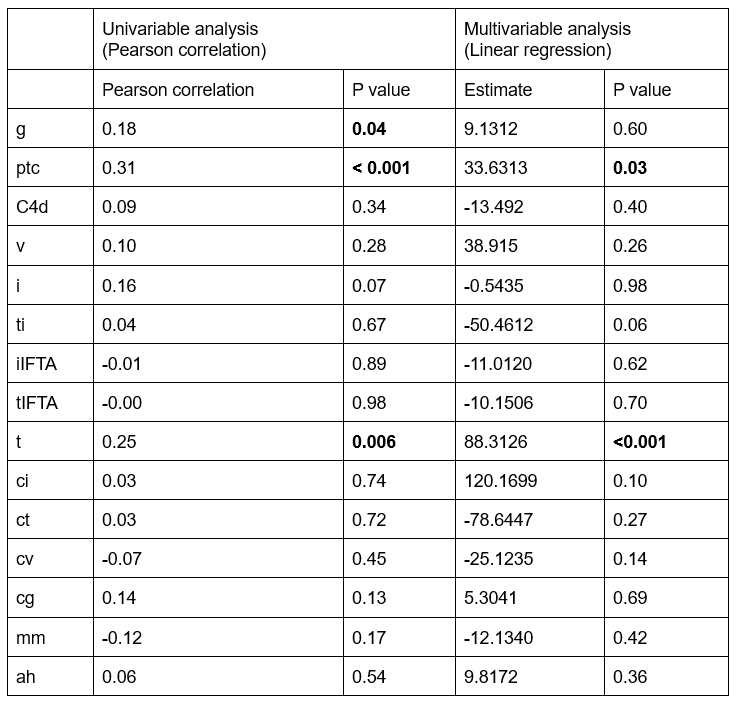
Results: In total, we examined 125 dd-cfDNA-paired biopsies from 110 KTR performed at our center between April 2020 and October 2023. Absolute levels of dd-cfDNA were significantly higher in mixed rejection (median 156 cp/mL, IQR 138-176), ABMR (median 63cp/mL, IQR 48-81), DSA-negative microvascular inflammation (DSAnegMVI) (median 74 cp/mL, IQR 35-134), and BK-virus associated nephropathy (BKVAN) (median 168cp/mL, IQR 54-311) in comparison to glomerulonephritis (median 12cp/mL, IQR 8-19), as well as in comparison CNI-toxicity (median 10, IQR 6-15). Additionally, patients with mixed rejection, ABMR, and BKVAN had higher absolute dd-cfDNA levels than biopsies with interstitial fibrosis and tubular atrophy (IFTA), as well as unremarkable histology. Absolute dd-cfDNA levels were higher in patients with mixed rejection than in those with UTI. In the univariable analysis, dd-cfDNA increase significantly correlated with the Banff scores for glomerulitis (g), peritubular capillaritis (ptc), and tubulitis (t). In the multivariable analysis, ptc and t were independently correlated with absolute dd-cfDNA levels.
Conclusion: Increase of dd-cfDNA is associated with kidney allograft damage beyond rejection and also occurs in alternative injury patterns with microvascular inflammation and severe tubulointerstitial inflammation, such as DSAnegMVI and BKVAN. Remarkably, dd-cfDNA levels remain low in the setting of de novo or recurrent glomerulonephritis and CNI-toxicity, which are important differential diagnoses in KTR undergoing clinical biopsy.
Laboratory testing was sponsored by Oncocyte.
[1] kidney transplantation
[2] allograft rejection
[3] noninvasive biomarker
[4] molecular diagnostics
[5] kidney allograft biopsy
[6] Banff classification
[7] microvascular injury
[8] glomerulitis
[9] nephropathology
