Longitudinal dd-cfDNA trends and clinical outcomes in kidney transplant recipients
Sumphami Bunnapradist1, Jonathan Bromberg2, Anthony Langone3, Tarek Alhamad4, Yen-An Chen5, Navchetan Kaur5, D. Giovanni Biagini5, Sangeeta Bhorade5, Adam Prewett5, Zachary P Demko5, Hossein Tabriziani5, Philippe Gauthier5, Matthew Cooper6.
1UCLA School of Medicine, Los Angeles, CA, United States; 2Department of Surgery, University of Maryland School of Medicine, Baltimore, MD, United States; 3Vanderbilt University Medical Center, Nashville, TN, United States; 4Division of Nephrology, Washington University, St. Louis, MO, United States; 5Natera, Inc., Austin, TX, United States; 6Medical College of Wisconsin, Milwaukee, WI, United States
The ProActive Study Investigators.
Introduction: Donor-derived cell free DNA (dd-cfDNA) is a biomarker of ongoing allograft injury and rejection used to monitor patients following kidney transplantation (Tx). It is hypothesized that dd-cfDNA may also be prognostic, and that persistently low dd-cfDNA levels indicate clinical stability while elevations in dd-cfDNA in the absence of rejection indicate immunological insult or impending rejection. Here, we assessed associations between longitudinal dd-cfDNA trends, pre-Tx immune status and outcomes in kidney-Tx patients with no rejection in the monitoring period.
Methods: Kidney Tx patients from a preliminary analysis of the ProActive (NCT04091984) study that had no biopsy proven rejection within 18 months of Tx and >3 dd-cfDNA tests (the ProsperaTM test, Natera, Austin, TX) performed between 90 days and 18 months post-Tx were included in the analysis. Patients were categorized into risk categories based on their pre-Tx metrics and into dd-cfDNA categories based on longitudinal trends (Figure 1). Patient outcomes were assessed through 24 months post-Tx. Associations between patient status at the time of Tx, dd-cfDNA trends and outcomes were analyzed.
Results: 370 patients with 2040 total dd-cfDNA tests were included. Patients were categorized by pre-Tx risk, dd-cfDNA trends and outcomes (Figure 1). The majority of patients (61.6%; 228/370) had dd-cfDNA levels consistently below 0.3% and another 18.9% (70/370) stayed below 0.5% (Table 1). Unremarkable pre-Tx risk status was associated with dd-cfDNA levels consistently below 0.3% (p=0.025). dd-cfDNA levels consistently below 0.3% were associated with a low incidence of adverse events, while elevated dd-cfDNA (highest dd-cfDNA result >0.5%) was associated with a high incidence of adverse outcomes, (p <0.0001). 35.9% of patients with a highest dd-cfDNA result between >0.5% and <0.99% had an adverse outcome, compared to 77.8% and 93.3% of patients with 1 and 2+ dd-cfDNA results >1%, respectively. Win ratio for patients below 0.3% dd-cfDNA was 2.48 (1.31-4.68).
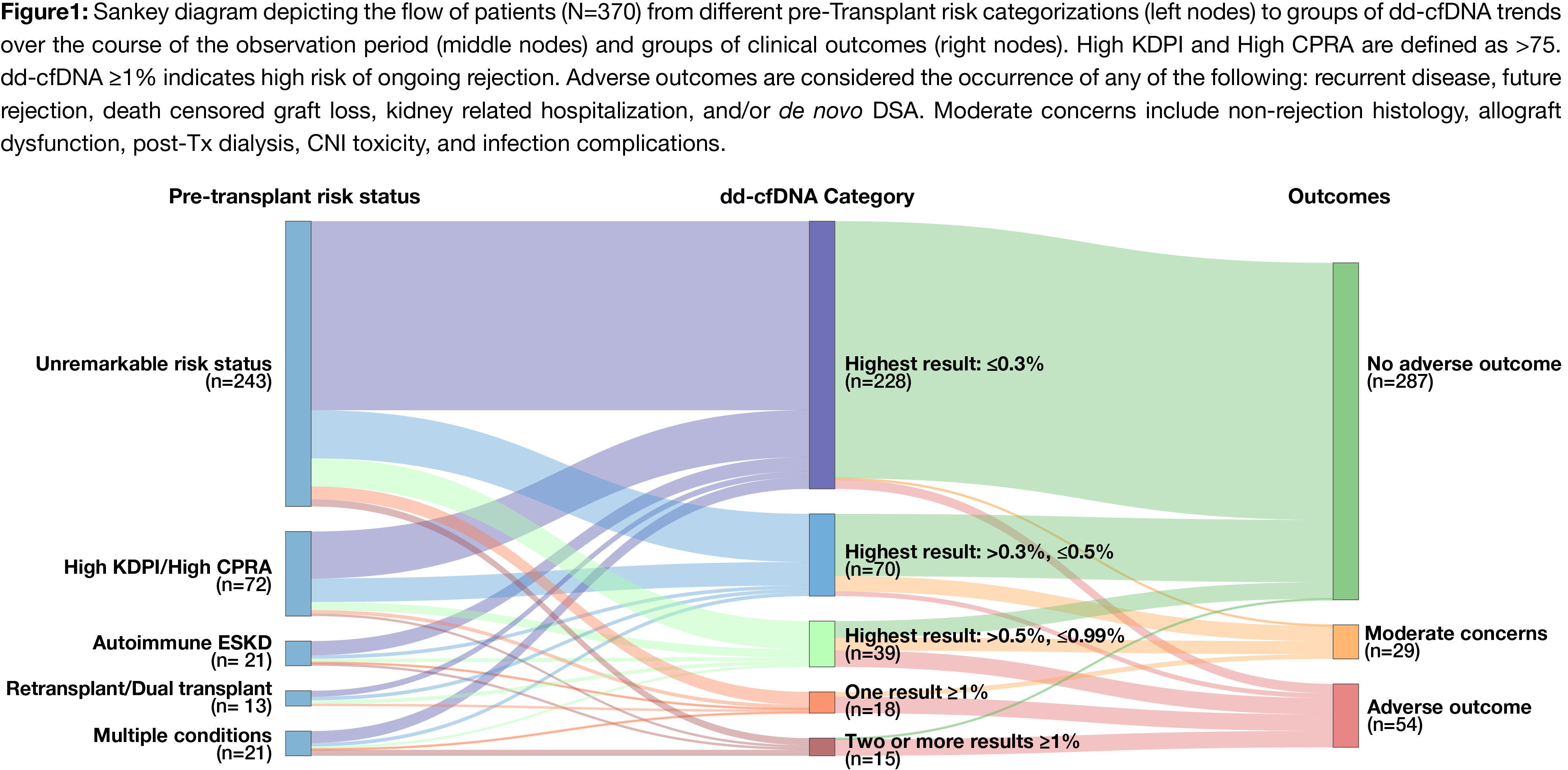
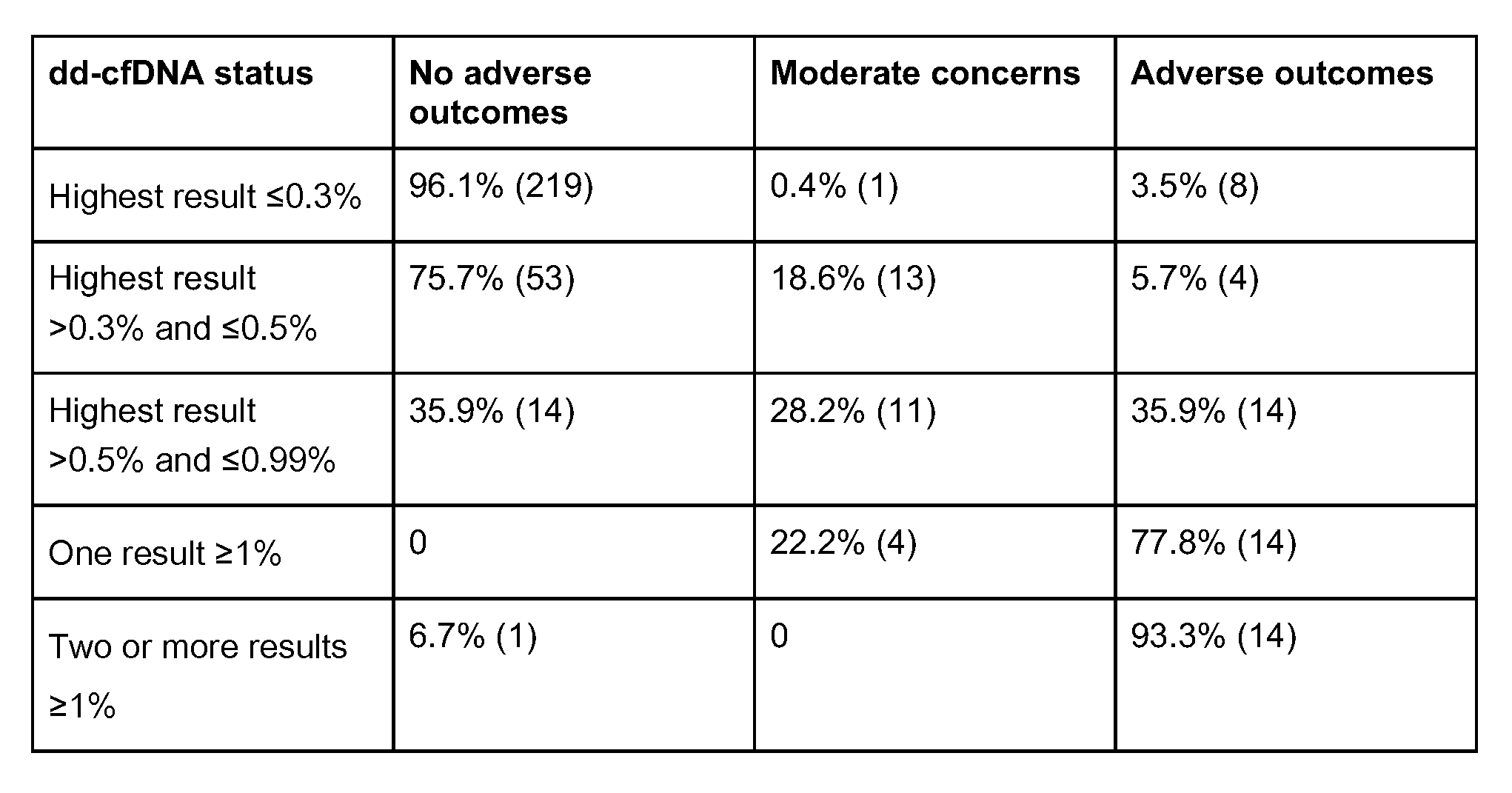
Conclusions: These findings suggest that dd-cfDNA trends may allow stratification of patients into different risk categories. Notably, dd-cfDNA levels consistently below 0.3% may be an indication of future clinical stability. Furthermore, the likelihood of adverse events increased with additional elevations in dd-cfDNA.
[1] dd-cfDNA
[2] Kidney Transplant
[3] Rejection
[4] Biomarker
