
Absolute dd-cfDNA monitoring reduces time to ABMR diagnosis in kidney transplant recipients with de novo DSA: a diagnostic randomized clinical trial
Aylin Akifova1, Klemens Budde MD1, Mira Choi MD1, Michael Oellerich MD2, Julia Beck PhD3, Kirsten Bornemann-Kolatzki PhD3, Ekkehard Schütz PhD3, Nils Lachmann Dr.4, Philip F. Halloran MD5, Bilgin Osmanodja MD1.
1Department of Nephrology and Intensive Care, Charité - Universitätsmedizin Berlin, Berlin, Germany; 2Department of Clinical Pharmacology, University Medical Center Göttingen, Göttingen, Germany; 3Chronix Biomedical GmbH, Göttingen, Germany; 4Centre for Tumor Medicine, Histocompatibility & Immunogenetics Laboratory, Charité - Universitätsmedizin Berlin, Berlin, Germany; 5University of Alberta, Edmonton, AB, Canada
Introduction: Donor-derived cell-free DNA (dd-cfDNA) is an emerging biomarker in transplantation that non-invasively detects graft pathologies with increased cellular damage, such as allograft rejection. Recent studies demonstrated its excellent diagnostic performance in detecting antibody-mediated rejection (ABMR) in kidney transplant recipients (KTR). However, the clinical benefits of routine dd-cfDNA monitoring still need to be established.
Methods: In this diagnostic, single-center, open-label, randomized clinical trial, we assigned 40 KTR with prevalent de novo DSA (dnDSA) with mean fluoerscence intensity (MFI) >1000 and estimated glomerular filtration rate ≥20 mL/min/1.73m2, but without biopsy-proven ABMR, to either dd-cfDNA-guided biopsy (intervention group) or clinician-guided biopsy (control group) over a 12-months period. In both groups, dd-cfDNA was assessed at inclusion and 1, 3, 6, 9, and 12 months. In the intervention group, dd-cfDNA >50cp/mL indicated a diagnostic biopsy. Biopsies for clinical indication could be performed at any point during the study period in both groups. A protocol biopsy was scheduled after 12 months for patients without dd-cfDNA-guided biopsy or clinical indication biopsy until study completion. Along with conventional histopathological examination, additional analysis with the Molecular MicroscopeⓇ Diagnostic System (MMDx) was available to assess the correlation with dd-cfDNA. The primary endpoint was time from study inclusion to diagnosis of active or chronic active ABMR.
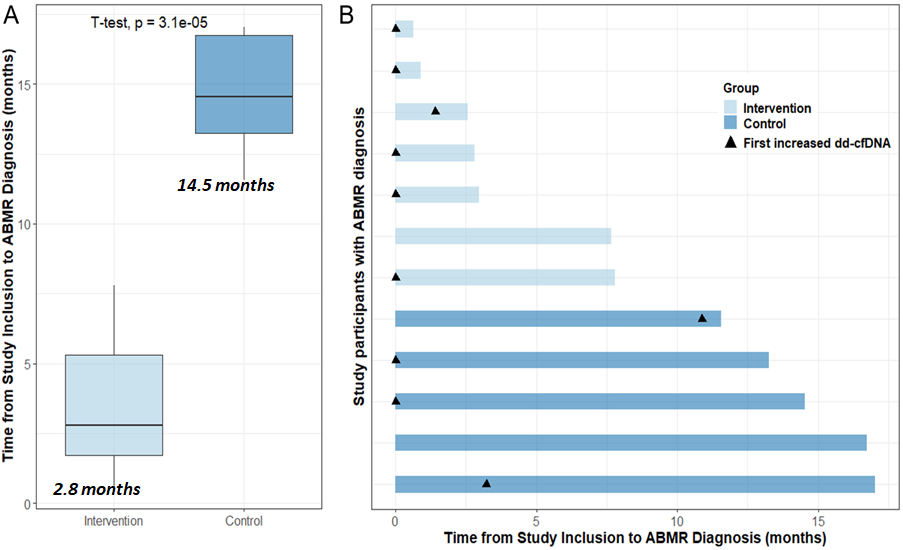
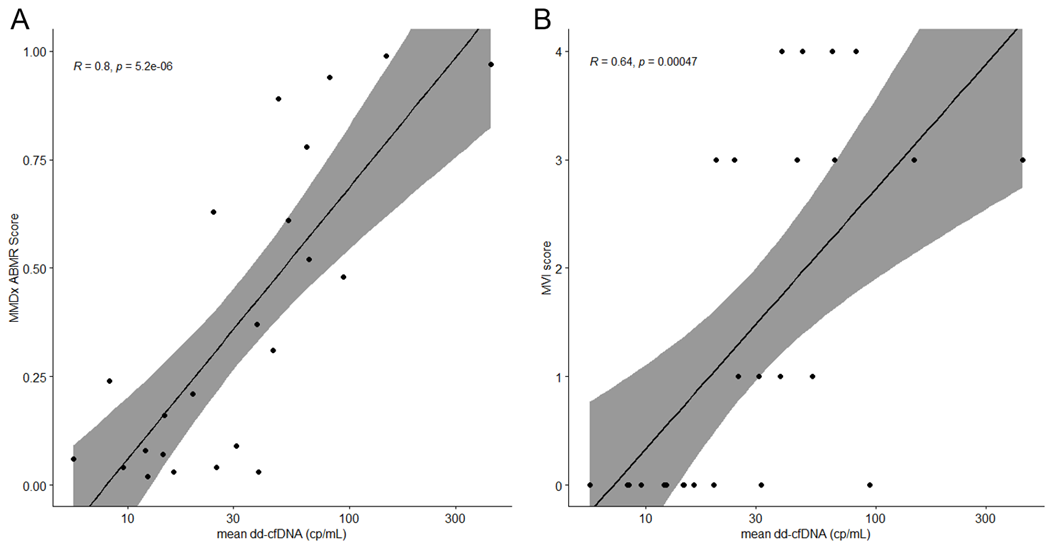
Results: 39/40 patients had functioning grafts at study completion. From these, 26 patients underwent biopsy, 13 in each group. ABMR was diagnosed earlier in the intervention group than in the control group (median 2.8 months, IQR 1.7-5.3 vs. median 14.5 months, IQR 13.3-16.7, p=0.003). Longitudinal dd-cfDNA monitoring with an absolute cutoff of 50 copies/mL had following test metrics for the detection of ABMR in DSA+ patients: AUC, 0.92; sensitivity, 0.83; specificity, 0.79; PPV, 0.77; NPV, 0.85, and was superior to routine biomarkers such as serum creatinine, urine albumin and dnDSA-MFI for distinguishing ABMR. Furthermore, absolute dd-cfDNA levels showed strong correlation (R=0.8, p<0.001) with the molecular ABMR score in MMDx and moderate correlation (R=0.64, p<0.001) with ABMR features, such as microvascular inflammation (g+ptc), in conventional histopathology.
Conclusion: We conclude that dd-cfDNA-guided biopsy in KTR with prevalent dnDSA can reduce the time to ABMR diagnosis and hereby expedite therapy initiation.
Laboratory testing was sponsored by Oncocyte.
[1] kidney transplantation
[2] allograft rejection
[3] antibody-mediated rejection
[4] donor-derived cell-free DNA
[5] donor-specific HLA antibodies
[6] molecular diagnostic techniques
[7] randomized controlled trial
[8] noninvasive biomarkers
