Dhakshayini Tharmaraj, Australia has been granted the TTS Scientific Congress Award

Dr. Dhaksha Tharmaraj is a Nephrologist at Monash Medical Centre, Australia and is currently completing her PhD in transplantation through Monash University. Her thesis examines the net state of immunity and the complex relationship between infection and rejection in transplantation. Prior to pursing medicine she completed a Bachelor of Radiography and Medical Imaging degree at Monash University. She has particular interests in transplant medicine, immunobiology and the application of medical imaging in Nephrology.
Intragraft gene transcript changes with intravenous immunoglobulin therapy versus standard care in chronic antibody mediated rejection- vipar study cohort
Dhakshayini Tharmaraj1,2, Greg Tesch1,2, Moshe Olshansky3, Kevan R Polkinghorne1,2,4, Sukhpal Dayan5, Edward Kwan5, Tia Mark1, John Kanellis1,2, David Nikolic-Paterson1,2, William R Mulley1,2.
1Department of Nephrology, Monash Medical Centre, Clayton, Australia; 2Department of Medicine, Monash University , Clayton, Australia; 3Monash Genomics and Bioinformatics, Monash University , Clayton, Australia; 4Department of Epidemiology and Preventative Medicine, Monash University, Clayton, Australia; 5Department of Anatomical Pathology, Monash Medical Centre, Clayton, Australia
VIPAR study group - Darren Lee, Peter Mount, Germaine Wong, Kate Wyburn, Wai Lim, Peter Kerr .
Introduction: Chronic antibody mediated rejection (cAMR) is a leading cause of graft loss, with limited treatment options with proven efficacy. Intravenous immunoglobulin (IVIg) has pleiotropic immunomodulatory effects and is a commonly adopted therapy in cAMR. Intragraft gene transcripts have prognostic significance in antibody mediated rejection. Mechanisms of action of IVIg and its impact on gene expression in cAMR is unknown. Our study aimed to identify intragraft gene transcript changes associated with IVIg treatment and shed light on its mechanistic impact in cAMR.
Methods: The VIPAR study compared clinical and histological outcomes in 30 kidney transplant recipients (KTRs) with cAMR randomised 1:1 to 6 months of IVIg versus no-IVIg. mRNA extracted from allograft biopsies taken at 0, 3, 6 and 12-months post-diagnosis was analysed using NanoString (B-HOT panel). Gene transcript differential expression (DE) was assessed using paired and time-course analyses. DE was defined as >1.5x gene fold change (FC) between groups with an adjusted p-value <0.05.
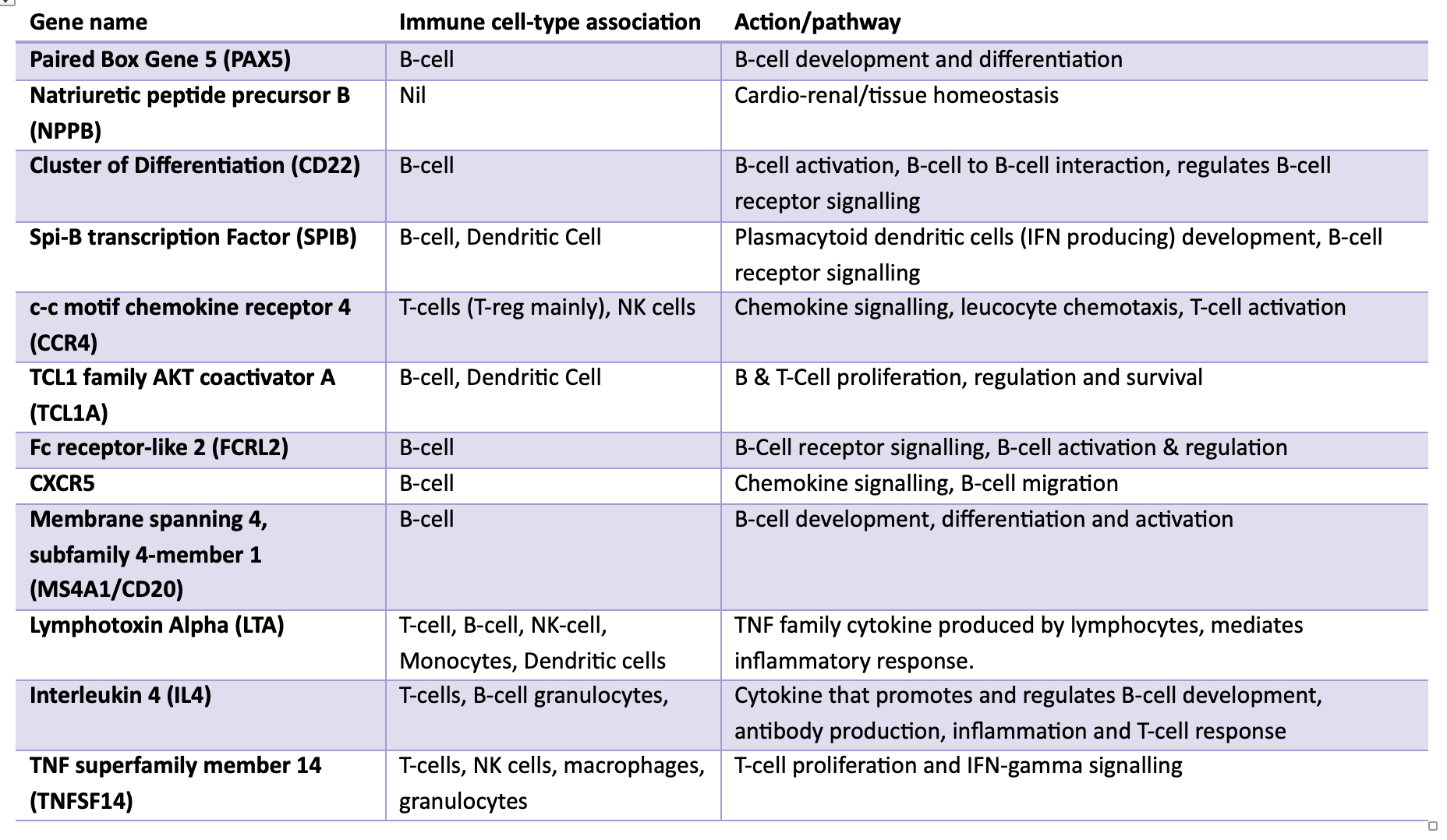
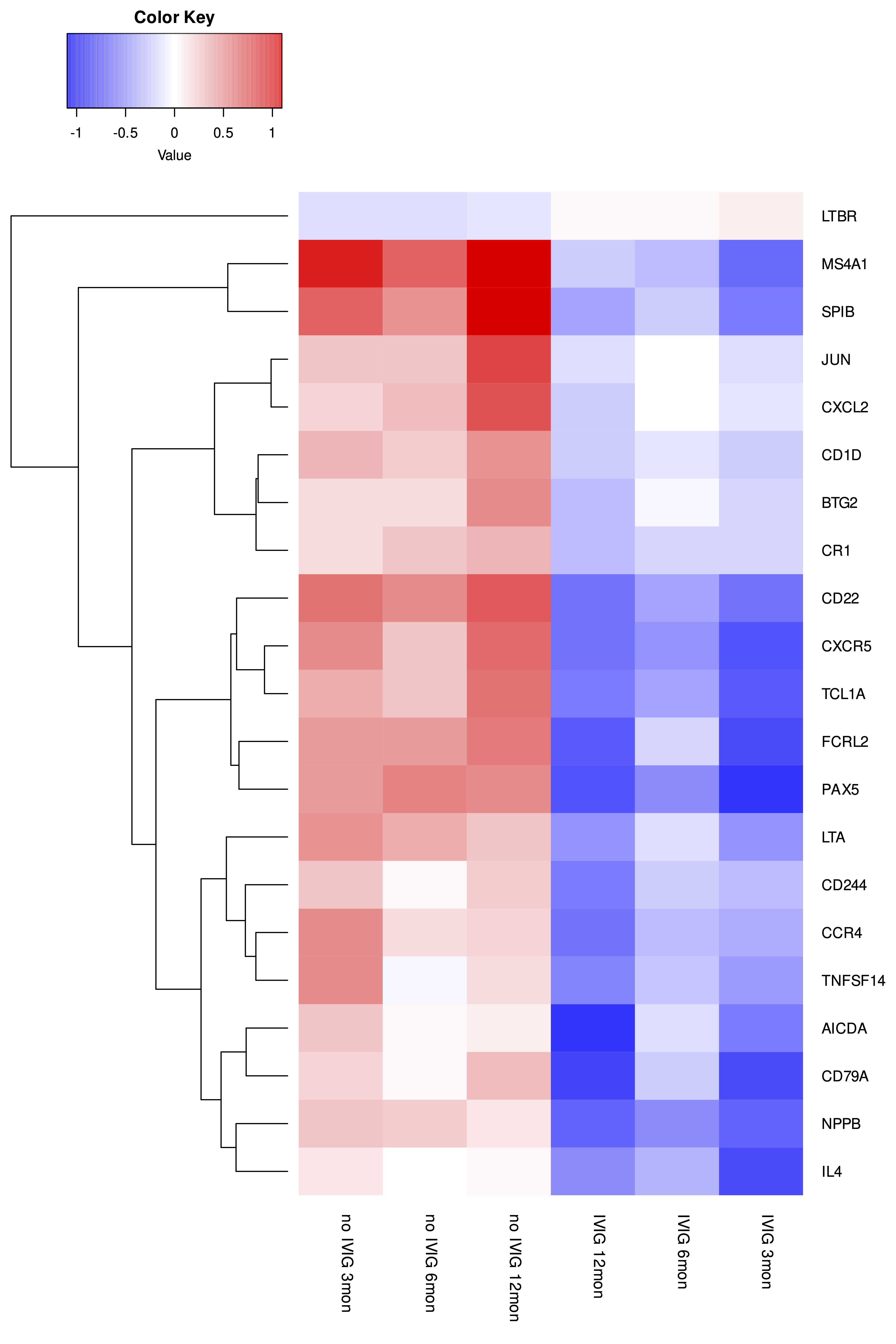
Results: The VIPAR study demonstrated reduced deterioration in chronic allograft injury and eGFR with IVIg relative to no-IVIg. Paired analysis (combined 3, 6, 12-month timepoints) revealed 59 downregulated genes in allografts from IVIg-treated relative to no-IVIg-treated patients. Nine of the top 12 DE genes were B-cell related (Table). These genes were most commonly associated with haematopoiesis (including B-cell development and differentiation), adaptive immunity and T-cell receptor signalling pathways. 12-month time-course analyses demonstrated different profiles for the IVIg relative to the no-IVIg group for 21 genes, with one increasing (LTBR) and 20 decreasing including B-cell (PAX5, CD22, SPIB, FCRL2, MS4A1, AICDA, TCL1A, IL4), T-cell/NK cell (CCR4, TNFSF14, CD244, IL4), pro-inflammatory (LTA, CXCL2) and pro-fibrotic (JUN) transcripts (Figure). Whilst 13 potentially injurious genes were upregulated in the timecourse analysis of the no-IVIg group, 22 genes were significantly downregulated in the IVIg group. There were no significant differences in gene transcripts in KTRs who subsequently developed graft failure in either group compared to those who did not.
Conclusion: Whilst IVIg had the greatest impact on B-cell associated gene expression, T-cell, pro-inflammatory and pro-fibrotic gene expression were also significantly altered. These findings provide mechanistic insights into the pleiotropic effects of IVIg and its potential therapeutic benefits in cAMR.
Monash Genomics and Bioinformatics Platform.
[1] Chronic antibody mediated rejection
[2] Intravenous immunoglobulin
[3] Gene transcripts
[4] mRNA
[5] graft rejection
[6] IVIG
[7] Gene expression
