Outcomes of simultaneous liver kidney transplant recipients according to pre-transplant transjugular intrahepatic portosystemic shunt (TIPS) in the United States
Tristan Meier1, Kathryn Schmidt2, Kristin Cole3, Jody Olson2, Timucin Taner5, Douglas A Simonetto2, Samy Riad4.
1Mayo Clinic Alix School of Medicine, Mayo Clinic, Rochester , MN, United States; 2Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States; 3Division of Clinical Trials and Biostatistics, Mayo Clinic, Rochester, MN, United States; 4Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, United States; 5Department of Surgery , Mayo Clinic, Rochester, MN, United States
Background: Previous data from a single center suggested that the outcomes for liver-alone recipients following transjugular intrahepatic portosystemic shunt (TIPS) were comparable to those without TIPS. This study aims to investigate the association between TIPS and outcomes among simultaneous liver-kidney (SLK) recipients nationally in the United States.
Methods: Utilizing the Scientific Registry of Transplant Recipients (SRTR) standard analysis file from 2003 to 2022, we examined 9,717 adult SLK recipients, among whom 858 had undergone TIPS before transplantation. Kaplan-Meier curves were generated to assess recipient, death-censored liver, and kidney graft survival. Mixed effects Cox proportional hazard models were employed to analyze the association between TIPS and the outcomes of interest, where transplant center was treated as a random effect. The models were adjusted for various factors, including recipient age, sex, MELD score, diabetes, duration of listing, induction, steroid maintenance, hepatitis C status, donor age, sex, cold ischemia time, local vs. shipped organs, and allocation era.
Results: Overall, the two groups were comparable, with minor differences. Notably, the median liver waiting time was significantly longer in the TIPS group compared to the non-TIPS group (4.1 vs. 2 months, p<0.001). One-year rejection rates for liver and kidney allografts did not differ significantly between the groups (Table 1). Univariable Kaplan-Meier analyses demonstrated no association between TIPS and worse outcomes for recipient, liver, and kidney survival (p=0.65, p=0.22, and p=0.54, respectively) (Figure 1). TIPS did not emerge as a predictor of recipient, death-censored liver, or kidney graft survival in multivariable models (Table 2).
Conclusion: In this extensive national cohort of SLK transplant recipients, pre-transplant TIPS was not linked to adverse outcomes for recipients or their allografts.
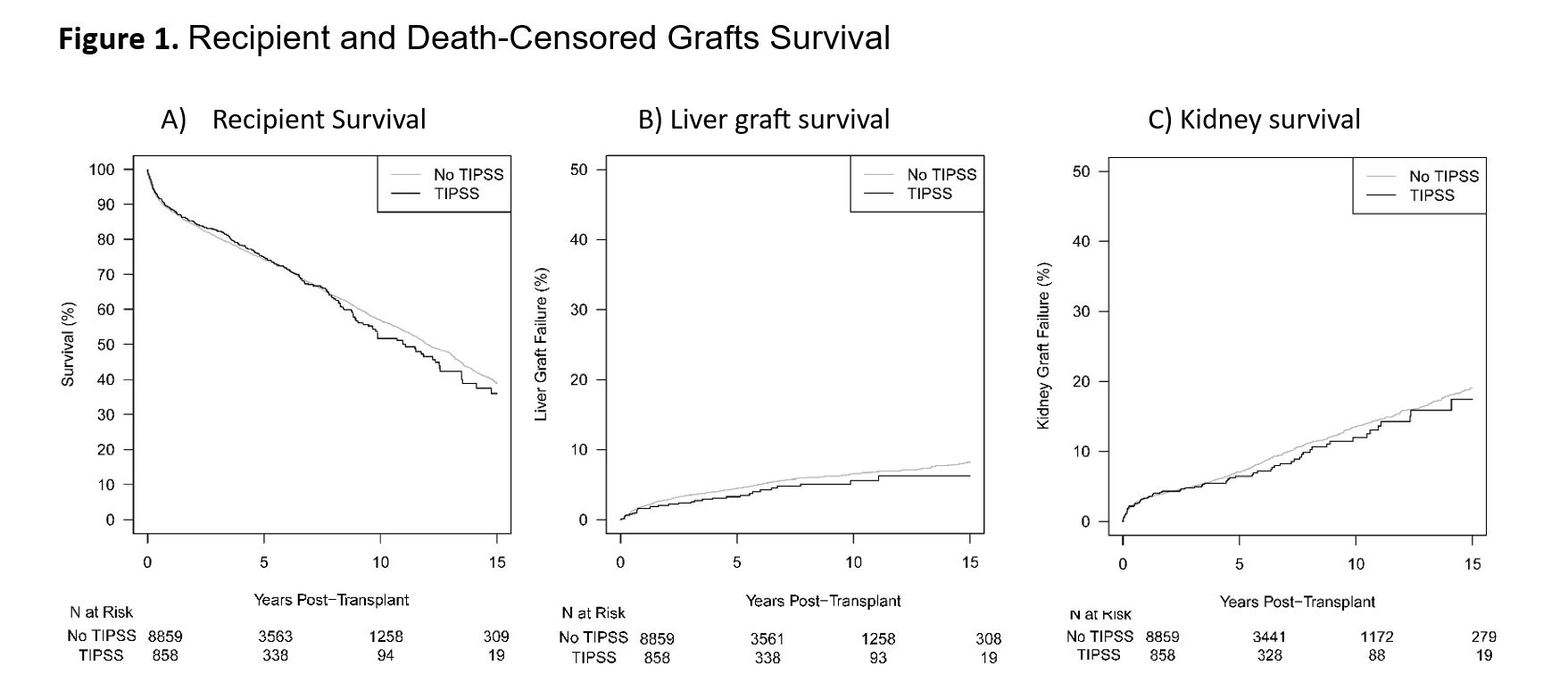
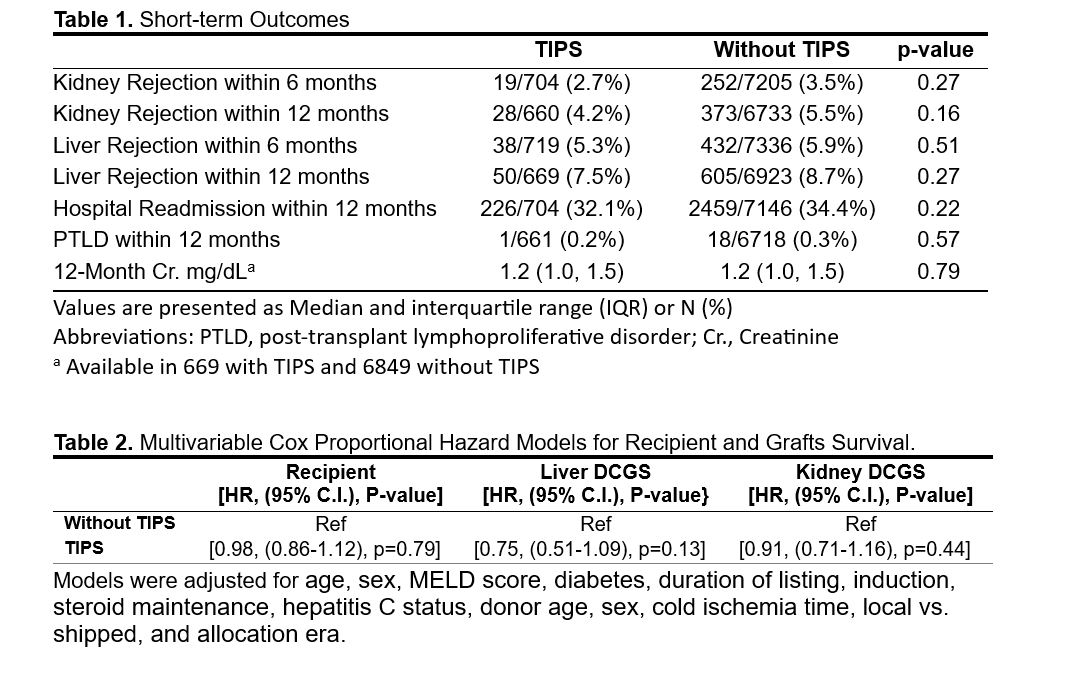
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
[1] TIPS
[2] SLK
[3] Outcomes
