Enver Akalin, MD, earned his medical degree at Ege University School of Medicine in Izmir, Turkey. He completed a research fellowship in the Renal Division at Brigham and Woman’s Hospital, Harvard Medical School, followed by a clinical fellowship in the Renal Division at Boston University School of Medicine. Dr. Akalin completed his residency in Internal Medicine as well as a clinical transplant nephrology fellowship in the Renal Division at Emory University School of Medicine, Atlanta.
After completing his fellowship, Dr. Akalin joined the Mount Sinai School of Medicine as a faculty in 2000 and served as the Medical Director of the Kidney and Pancreas Transplantation Program until January 2009. He is currently the Medical Director of the Kidney Transplantation Program at Montefiore Medical Center /Albert Einstein College of Medicine, Bronx, NY and Professor of Medicine and Surgery.
Dr. Akalin’s research interest is understanding the mechanisms of allograft injury. He is the first investigator who applied microarray technology in kidney transplant recipients. He has had involved in multiple NIH studies and currently has active RO1 grants. He involved in more than 30 multicenter clinical trials as a site principal investigator. He has authored or co-authored more than 135 original scientific articles, review articles and book chapters. He had served on the Editorial Board of the Clinical Journal of The American Society of Nephrology. He is currently the Associate Editor of Transplantation and Chief Section Editor of Frontiers in Transplantation.
Dr. Akalin chaired the American Society of Transplantation, Transplantation and Immunology Research Network, Clinical Science Grant Review Committee and Transplant Nephrology Fellowship Accreditation Committee, Fellow of American Society of Transplantation Committee, and the governing board of Transplant Nephrology Fellowship Training Accreditation Programs. He served at Education Committee of the Transplantation Society. He currently serves at Medical Directors forum of American Society of Transplantation. Dr. Akalin was the president of New York Society of Nephrology during 2016 and 2017 academic year.
Dr. Akalin is currently the fellow of American Society of Nephrology (FASN) and American Society of Transplantation (FAST).
Not all transplant glomerulopathy related to chronic antibody-mediated rejection
Enver Akalin1, Mariel Barbachan e Silva2, Sadia Mustofa1, Yi Bao1, Pilib Ó Broin2.
1Einstein/Montefiore Abdominal Transplant Program, Montefiore Medical Center, Bronx, NY, United States; 2School of Mathematical & Statistical Sciences, , University of Galway, Galway, Ireland
Purpose: Transplant glomerulopathy (TGP) with donor-specific anti-HLA antibodies (DSA) and C4d or microvascular inflammation (MVI) is classified as chronic active antibody-mediated rejection (CAMR). We aimed to analyze intragraft gene expression profiles of TGP without any DSA or C4d or MVI.
Methods: One transplant kidney biopsy core was collected into a vial containing 1 mL of RNALater and analyzed by Affymetrix Human Gene 1.0 ST Array hybridization (28 869 gene probe sets). A support vector machine (SVM) with recursive feature elimination (RFE) was used to identify gene signatures capable of classifying TGP samples from normal transplant kidney biopsies without any acute or chronic Banff Allograft injury scores (Benjamini-Hochberg adjusted p < 0.05). Selected genes were then used as input to the Enrichr pathway enrichment analysis tool. DSAs were studied by Luminex single antigen beads and mean fluorescence intensity value more than 1000 were accepted as positive.
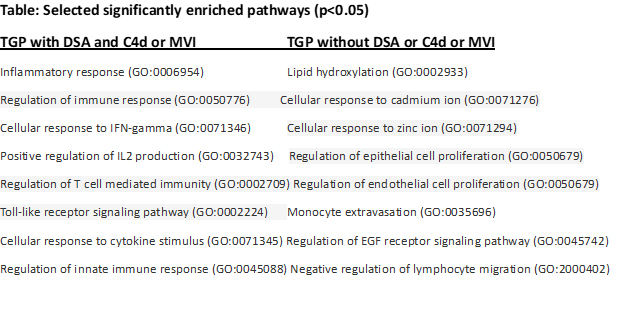
Results: Twenty-two TGP samples with DSA and C4d or MVI,10 TGP samples without any DSA or C4d or MVI, and 22 normal transplant kidney biopsy samples were included in analysis. Biopsies were done at a median 6.4 years (IQR, 3.3-9.8) after transplantation. Median serum creatinine level was 2.2 mg/dl (IQR, 1.7-3.1) and spot urine protein/creatinine 1.7 g/g (IQR, 0.6-3.0). 59% of patients were male, 65% black and 64% were deceased-donor kidney transplant recipients. Pathway enrichment analysis of RFE-selected genes demonstrated enrichment in 14 pathways that were mainly related to immune response, interferon-gamma, T cell mediated immunity, IL2 production, innate immunity and toll-like receptor signaling pathway as shown at the Table (p < 0.05). However, for intragraft gene expression profiles of TGP samples without any DSA or C4d or MVI, the RFE-selected genes did not show significantly enriched pathways related to immune activity as shown in TGP samples with DSA and C4d or MVI. Among the 30 enriched pathways were: lipid hydroxylation, positive regulation of epithelial and endothelial cell proliferation, positive regulation of epidermal growth factor receptor signaling pathway, negative regulation of lymphocyte migration and monocyte extravasation.
Conclusions: TGP samples without DSA or C4d, or MVI showed significantly different intragraft gene expression profiles not related to immune activity or CAMR. These results indicated that TGP is an end organ damage and could develop through different mechanisms and not all related to antibody-mediated mechanisms.