A randomised controlled trial of intravenous immunoglobulin (IVIg) compared with standard of care for the treatment of chronic active antibody mediated rejection (VIPAR)
William Mulley1,2, Dhakshayini Tharmaraj1,2, Kevan R Polkinghorne1,2,3, Greg H Tesch1,2, Sukhpal K Dayan4, Edward Kwan4, Darren Lee5,6,7, David J Nikolic-Paterson1,2, John Kanellis1,2.
1Department of Nephrology, Monash Medical Centre, Clayton, Australia; 2Centre for Inflammatory Diseases, Department of Medicine, Monash University, Clayton, Australia; 3Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Australia; 4Department of Anatomical Pathology, Monash Health, Clayton, Australia; 5Department of Renal Medicine, Eastern Health, Box Hill, Australia; 6Eastern Health Clinical School, Monash University, Clayton, Australia; 7Department of Nephrology, Austin Health, Heidelberg, Australia
VIPAR Study Group - Tia Mark, Peter Mount, Germaine Wong, Kate Wyburn, Wai Lim and Peter Kerr.
Introduction: Chronic active antibody mediated rejection (cAMR) is the leading death-censored cause of kidney allograft loss but has no proven effective treatments. IVIg is commonly used to treat cAMR but its efficacy and mechanisms of action are unknown. We aimed to determine if IVIg is an effective therapy for cAMR and to understand IVIg’s mechanisms of action in cAMR.
Methods: We conducted a multicentre randomised controlled trial, in patients with biopsy-proven cAMR, comparing 6 doses (1gm/kg/month) of IVIg (n=15) to no-IVIg (n=15) (Trial Registration: ACTRN12612000252819). All participants received pulse methylprednisolone and maintenance tacrolimus, mycophenolate and prednisolone. The primary outcome was change in chronic allograft damage index (CADI) scores over the first 12-months from enrolment. Secondary outcomes included: change in eGFR, change in donor specific antibodies (DSA) and graft and patient survival. Additionally, change in intragraft mRNA expression was assessed using the Nanostring B-HOT panel. All parameters were assessed at enrolment, 3-, 6- and 12-months post-diagnosis, while eGFR and graft and patient survival were also assessed at 2 years. Biopsies were scored by 2 renal histopathologists. Change over time was assessed with linear mixed models and survival by Cox proportional hazard models.
Results: At enrolment the mean age of participants was 56.1 years (SD 12.1), 22 were male and the mean eGFR was 41.0 ml/min/year (SD 13.6). Over the 12-months post-diagnosis CADI scores increased in the no-IVIg group and were stable in the IVIg group (Figure 1, interaction, p=0.003).
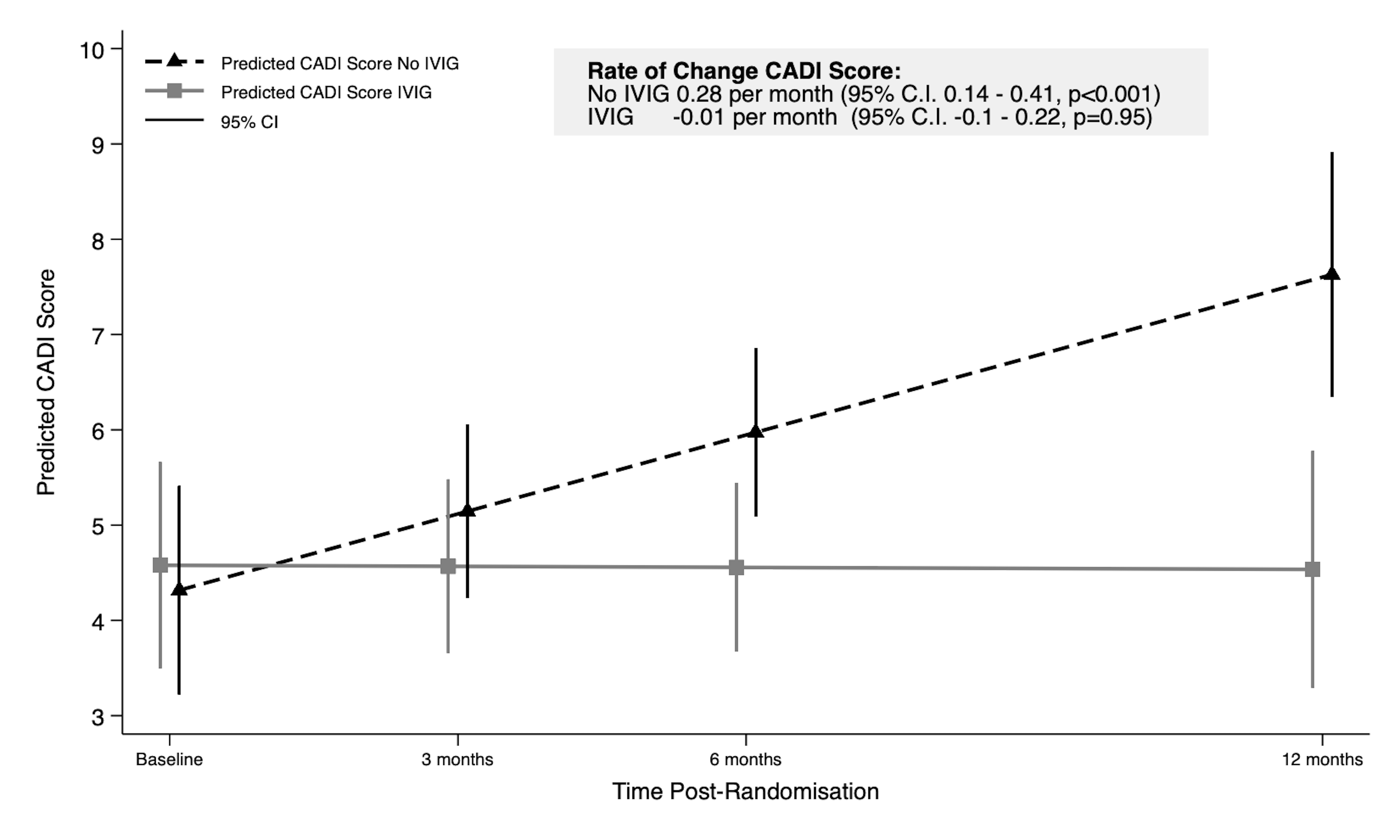
Over 2-years, eGFR declined more rapidly in the no-IVIg group (-10.1ml/min/year, 95% CI -13.2, -6.9) compared to the IVIg group (-3.6ml/min/year, 95% CI -6.5, -0.6, interaction p=0.003) (Figure 2).
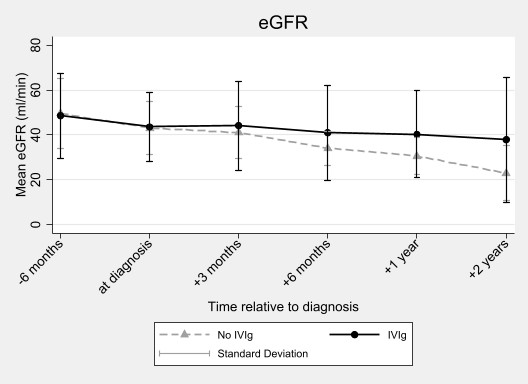
At 2 years post-diagnosis there were 3 participants with graft loss in the IVIg group and 5 in the no-IVIg group. There were 2 deaths in the no-IVIg group and none in the IVIg group. The change in the mean fluorescence indices of the immunodominant DSA were not different between groups. Intragraft expression of 59 genes (particularly B-cell related genes) reduced significantly with IVIg relative to no-IVIg.
Conclusions: IVIg therapy was associated with reduced deterioration in allograft injury and function in cAMR. This was not associated with reduced DSA production but may relate to intragraft effects of IVIg. Treatment with IVIg should be considered for patients with cAMR.
This work was supported by a grant from the Commonwealth of Australia as represented by the National Blood Authority. Grant number: IgP02.
[1] Antibody mediated rejection
[2] Randomised clinical trial
[3] intravenous immunoglobulin
[4] graft survival
[5] CADI score
