CCR5 Blockade: A novel, safe, and efficacious addition to the immunosuppressive armamentarium following kidney transplantation
Audrey Brown1, Burc Barin2, Shikha Mehta3, Sander Florman4, Christine Durand5, Thomas Pearson6, Matthew Cooper7, Valentina Stosor8, Peter Stock1.
1Transplant Surgery, University of California San Francisco, San Francisco, CA, United States; 2The Emmes Company, The Emmes Company, Rockville, MD, United States; 3Nephrology, University of Alabama at Birmingham, Birmingham, AL, United States; 4Transplant Surgery, Mount Sinai, New York, NY, United States; 5Infectious Disease, Johns Hopkins, Baltimore, MD, United States; 6Transplant Surgery, Emory, Atlanta, GA, United States; 7Transplant Surgery, Medical College of Wisconsin, Milwaukee, WI, United States; 8Infectious Disease, Northwestern, Chicago, IL, United States
NIH HIV TR CCR5 Consortium.
Introduction: Maraviroc (MVC) is a CCR5 blocker designed to inhibit entry of the HIV virus into the CD4+ T-cell. MVC also has immunomodulatory effects hypothesized to reduce rejection after kidney transplantation (KT) and has been approved for use in people with HIV for years. We tested the impact of adding MVC to immunosuppressive regimens on outcomes following KT in recipients with HIV (HIVR+). HIVR+ are an ideal population to test this hypothesis as they have a high incidence of rejection.
Methods: We performed a multi-center, double-blind, placebo-controlled, phase II randomized controlled trial (RCT) of HIVR+ to assess the safety and efficacy of adding CCR5 blockade to integrase-based antiretroviral therapy (ART) post-KT. The primary efficacy endpoint was glomerular filtration rate at 52 weeks. Safety and secondary endpoints are included in Table 1.
Results: 97 HIVR+ were randomized to MVC (n=49) versus placebo (n=48). eGFR (CKD-EPI) at one year was significantly higher in HIVR+ randomized to MVC versus placebo (mean of 59.2 versus 49.3 mL/min/1.73m2, p=0.026, Figure 1). The incidence of biopsy proven rejection was low and not significantly different between MVC (8.7%) and placebo (12.8%) arms (p=0.47). There were no differences in measures of inflammation on protocol biopsy at 6 months or tacrolimus trough levels at one month and one year (Table 1). There were no persistent virologic failures and no statistically significant differences in measures of the HIV reservoir (HIV DNA or RNA in CD4+ T cells). In terms of safety profile, there was no significant difference in the incidence of the composite primary safety endpoint (graft loss, > Grade 3 AE and/or permanent treatment discontinuation within the first 52 weeks post-transplant (log-rank test, p=0.545) between the treatment and placebo cohorts. No recipients developed HIV nephropathy.
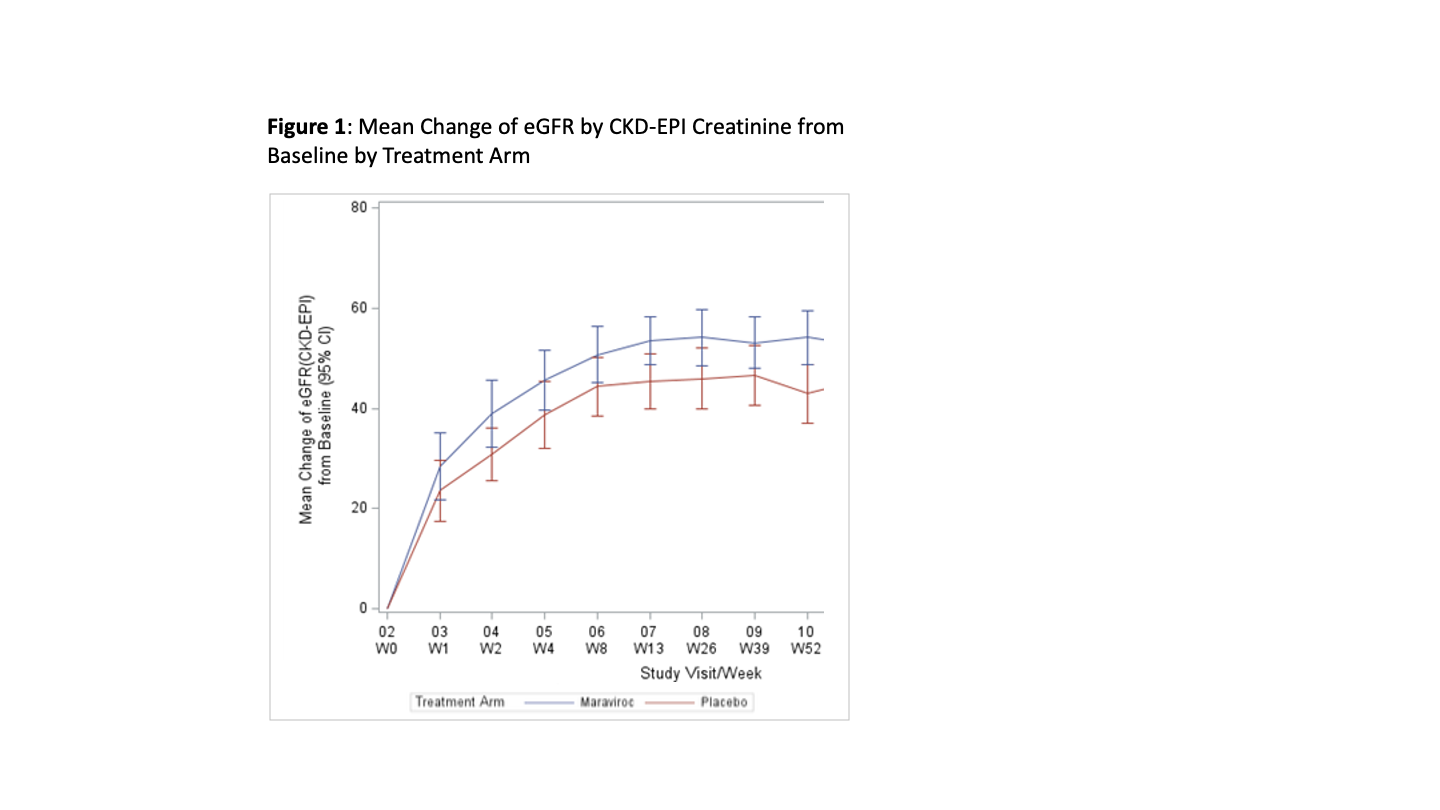
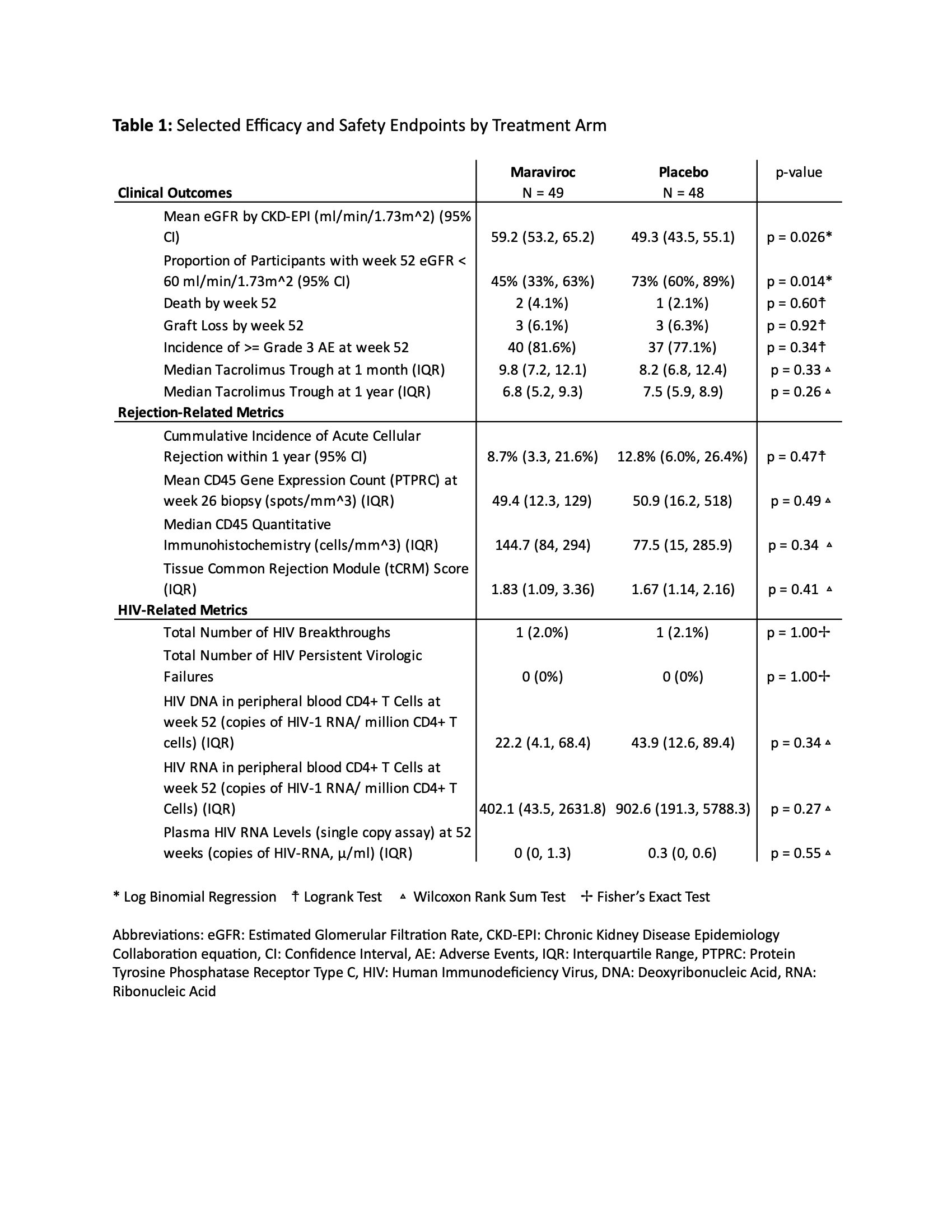
Conclusions: The addition of MVC to the immunosuppressive regimens following KT in HIVR+ resulted in a highly significant improvement (~10 ml/min/1.73m2) in eGFR at 52 weeks compared to placebo. This novel use of a well-tolerated blocker of the CCR5 receptor warrants further exploration in all KT recipients regardless of HIV status based on this exceptional improvement in eGFR at one year following KT in this RCT.
[1] Immunosuppression
[2] Human Immunodeficiency Virus
[3] CCR5 Blockade
[4] Acute Rejection
[5] Clinical Trial
[6] HIV Nephropathy
