A review on the roles of mTOR inhibitors in pediatric liver transplant recipients
Simin Dahti-Khavidaki1, Marjan Moghadamnia3, Hossein Alimadadi2.
1Liver Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran; 2Department of Gastroenterology, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran; 3Department of Clinical Pharmacy, Kermanshah University of Medical Sciences, Kermanshah, Iran
Aim: Considering the immunopharmacological properties of mammalian target of rapamycin (mTOR) inhibitors, such as their anti-inflammatory, anti-fibrotic, and antiproliferative effects, this review assesses the potential roles of this immunosuppressive class among pediatric liver transplant patients.
Method: A systematic literature survey was done to explore the utilization of mTOR inhibitors in various aspects of treatment of pediatric liver transplant recipients.
Results: The beneficial impacts of switching from calcineurin inhibitor (CNI) to mTOR inhibitor or adding mTOR inhibitor to CNI-reduced immunosuppression of pediatric liver transplant recipients with impaired kidney function is controversial and needs high-powered clinical studies. It appears that enhancing the immunosuppression through the addition of an mTOR inhibitor to CNI is helpful for pediatric liver transplant recipients who are experiencing refractory acute rejection or chronic rejection. mTOR inhibitor-containing regimens failed to reduce PTLD happening among children with liver transplantation that may be due to concomitant high CNI concentration among studied patients. The effectiveness of mTOR inhibitors in treating PTLD remains uncertain, however, in patients with PTLD who are at high risk of rejection, mTOR inhibitors may be administered. Conversion to or adding mTOR inhibitors to maintenance immunosuppression seems to be suitable for pediatrics who are transplanted due to hepatic malignancies such as hepatoblastoma or hepatocellular carcinoma or for those with post-transplant primary or recurrent malignancies. Switching to mTOR inhibitor may improve some CNI-related adverse effects such as gingival hyperplasia, neurotoxicity, nephropathy, hypertrophic cardiomyopathy, or thrombotic microangiopathy. Although there are some successful reports, further studies are needed to fully understand potential benefits of mTOR inhibitors in managing post-transplant de novo allergies or immune-mediated disorders.
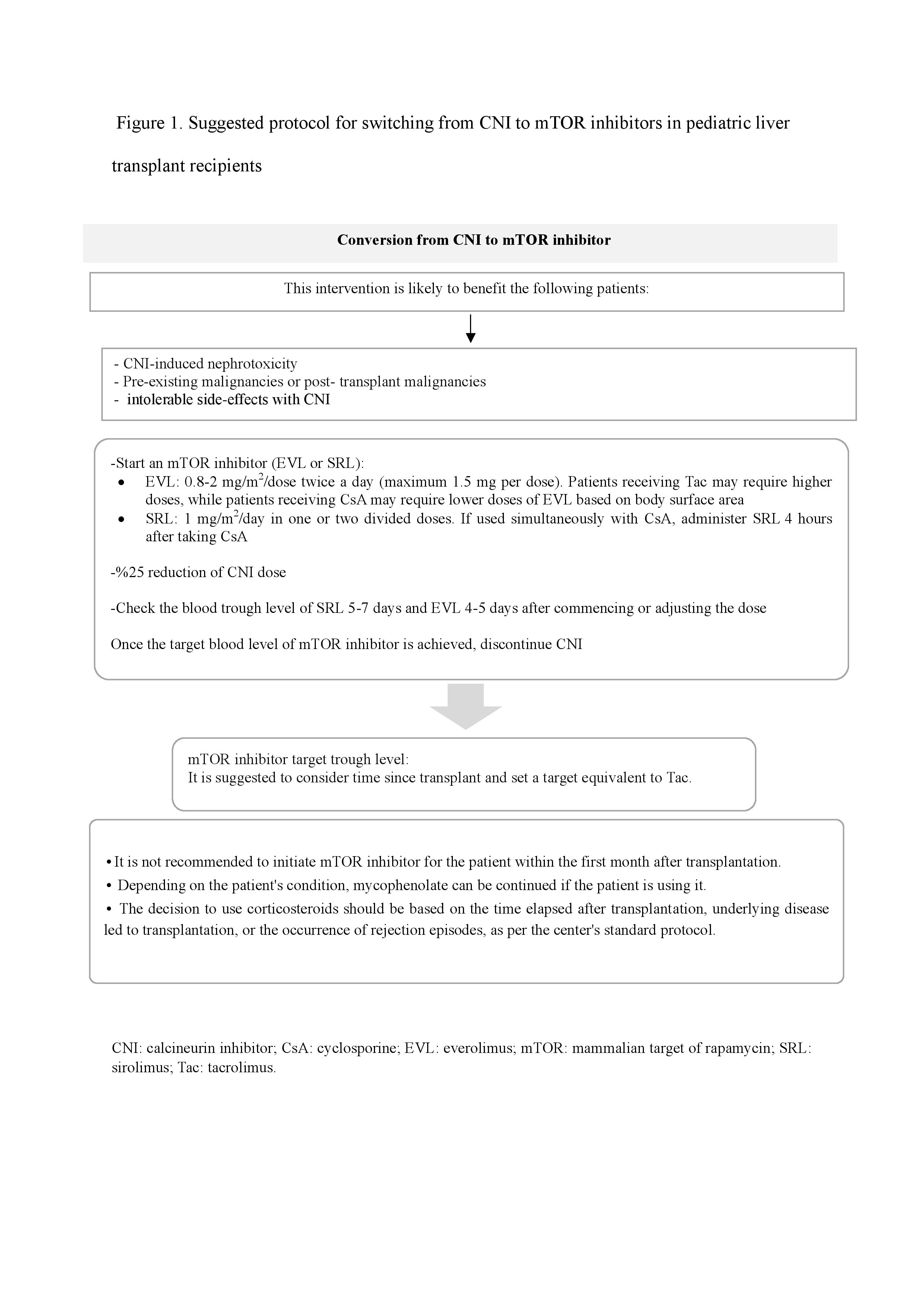
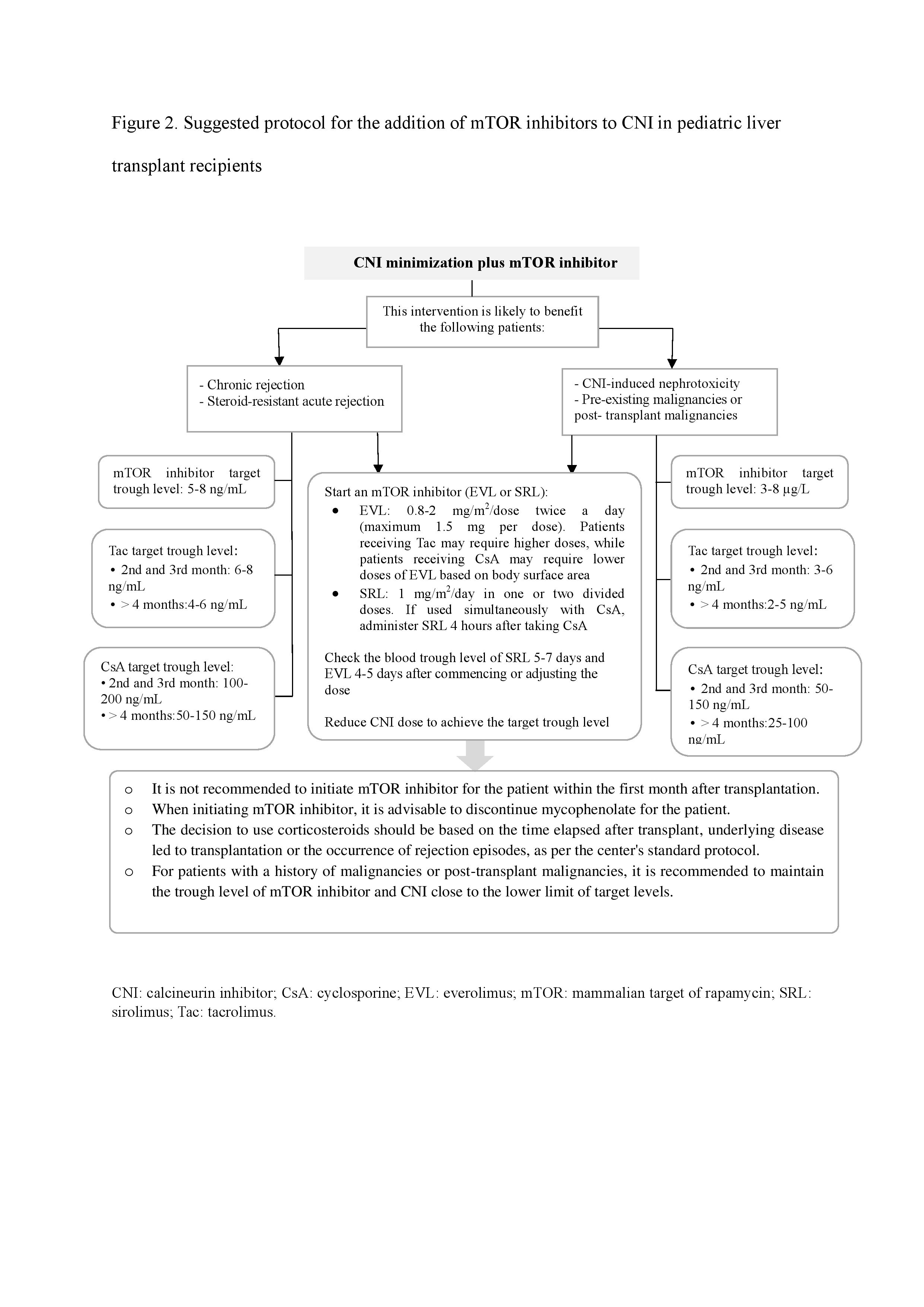
Conclusion: Although the exact role of mTOR inhibitors among pediatric liver transplant patients needs further study, two algorithms are presented in this review to guide converting from CNIs to mTOR inhibitors or adding mTOR inhibitor to a CNI-minimization immunosuppressive regimen for pediatrics that may benefit this class of drugs.
[1] Pediatrics
[2] Liver transplantation
[3] mTOR inhibitor
[4] Immunosuppressive therapy
