
Case studies in renal xenotransplantation pharmacology: pharmacokinetics and pharmacodynamics of tacrolimus, mycophenolate mofetil, and vancomycin
Karen Khalil1, Jacqueline I Kim1, Rebecca Dieter1, Ian Jaffe1, Imad Aljabban1, Brendan Keating1, Sapna Mehta1, Adam Griesemer1, Robert A Montgomery1, Jeffrey M Stern1.
1Transplant Institute, NYU Langone Health, New York, NY, United States
NYU Xenotransplant Research Group.
Introduction: The pharmacokinetics (PK) of medications in the setting of a porcine xenokidney are not well understood. In this study, we studied the PK of tacrolimus, mycophenolate mofetil (MMF), and vancomycin in a brain-dead decedent with an alpha-Gal knockout xenothymokidney.
Methods: A nephrectomized decedent received a porcine xenothymokidney transplant and was observed for 61 days. A standard maintenance immunosuppressive regimen was used. Pharmacokinetics of IV MMF 2 grams/day over 2 hours and tacrolimus, sublingual and suspension via jejunostomy tube, were calculated. Daily whole blood trough concentrations were drawn for tacrolimus. PK of MMF were obtained on POD 7 (Dose 1), 18 (Dose 2) and 41 (Dose 3). The decedent was maintained on intravenous vancomycin for skin/soft tissue infection prophylaxis, with trough concentrations analyzed every 48 to 72 hours or if there was a change in renal function.
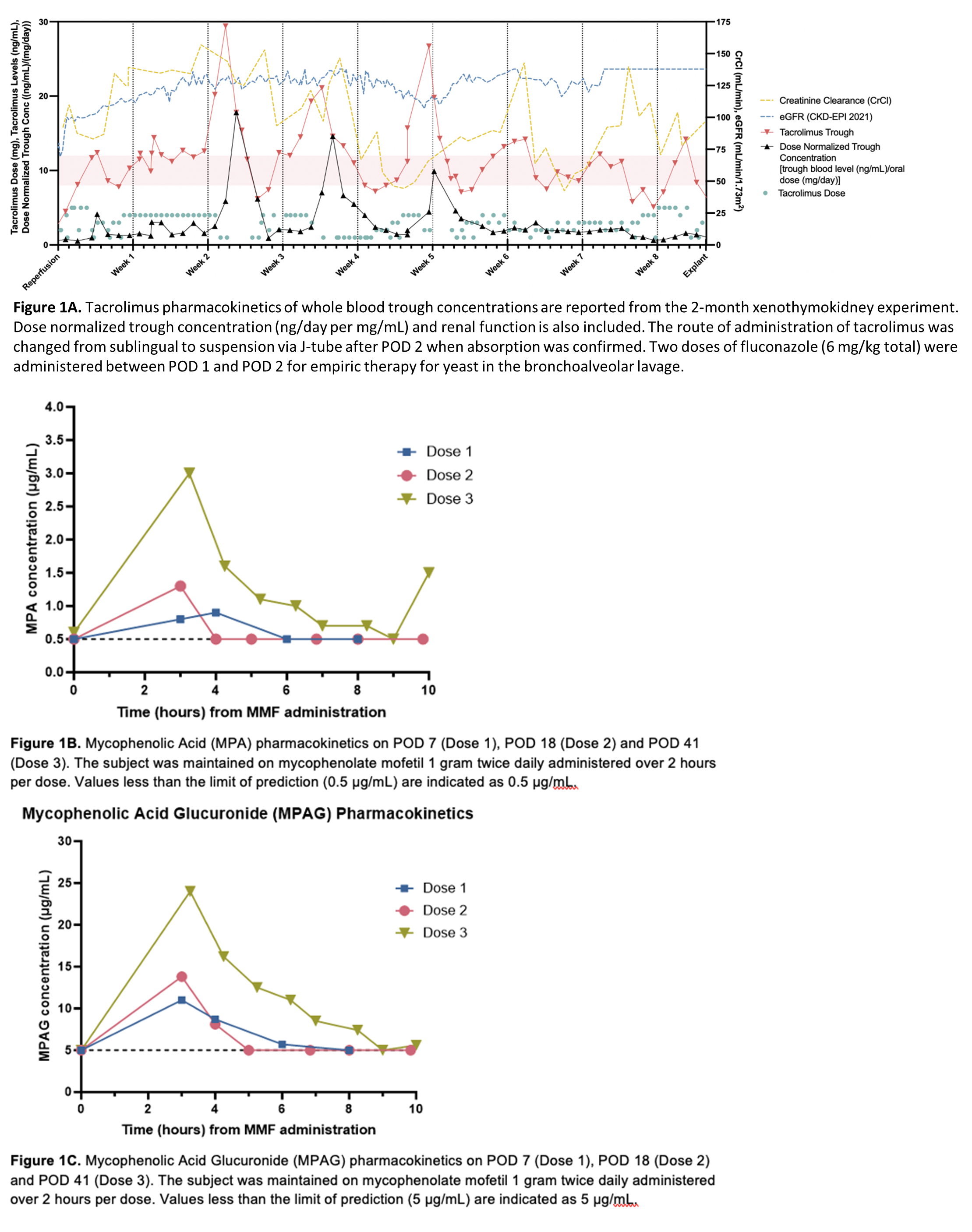
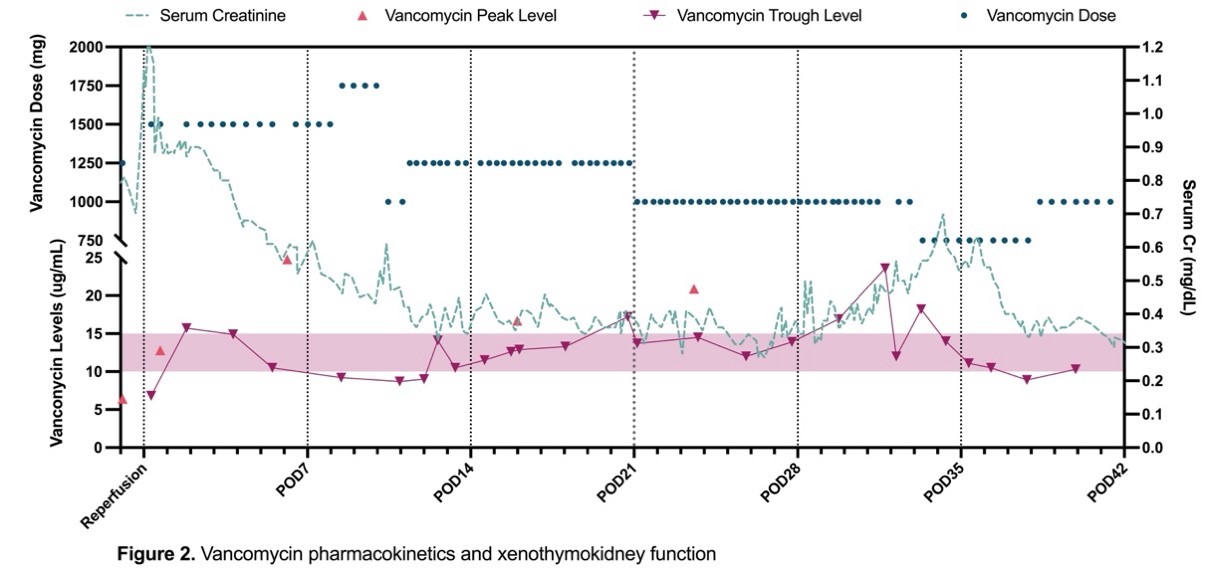
Results: Tacrolimus PK demonstrated variability in trough concentrations (Fig 1A) with a time within therapeutic range of 38%. The average dose-normalized trough concentration for tacrolimus at steady state was 2.9 ng/day per mg/mL. Variabilities in serum levels were attributed to changes in absorption, enteral feeds, and diarrhea due to Norovirus diagnosed on POD 50. Mycophenolic acid (MPA) AUCs by linear trapezoidal method using plasma concentrations were 2.7, 3.8, and 10.7 μg⋅h/mL for Doses 1, 2 and 3 (Fig 1B). MPA AUC was lower than the target range of 30-60 μg⋅h/mL despite standard doses, potentially due to induction of UGTs by high corticosteroid doses, differences in pig and human glucuronidation, and enterohepatic circulation. Mycophenolic acid glucuronide (MPAG) AUC (30-96 μg⋅h/mL) was lower than anticipated possibly due to a lower capacity for glucuronidation of MPA or hyperfiltration and rapid clearance by the xenothymokidney (Fig 1C). The vancomycin dose and frequency required to reach target concentrations (10-15 µg/mL) increased as serum creatinine and creatinine clearance improved (Fig 2). On POD 11, the calculated elimination constant was 0.123/hr and the half-life was 5.64 hr, which supported increased dose frequency. No evidence of vancomycin-induced kidney injury was seen clinically or on histology.
Conclusions: Tacrolimus PK in a decedent after xenothymokidney transplantation demonstrate variability likely due to absorption changes, diarrhea, and CYP3A4 drug interactions. MPA and MPAG AUCs were lower than expected possibly due to intermittent high dose corticosteroids, xenograft hyperfiltration, and other subject-specific factors. Vancomycin trough concentrations were maintained at goal, indicating effective excretion via glomerular filtration without any evidence of vancomycin-induced kidney injury or nephrotoxicity. Further research is necessary to characterize the PK, optimize therapeutic concentrations, and define the optimal dose of immunosuppressive and other medications after xenotransplantation.
Funding for this study was provided by the United Therapeutics Corporation.
[1] tacrolimus
[2] mycophenolate
[3] vancomycin
[4] pharmacokinetics
[5] immunosuppression
[6] xenotransplantation
[7] kidney transplant
