In vivo evaluation of pig kidney xenograft physiology: Water handling and the renin-angiotensin-aldosterone system
Vasishta Tatapudi1,2, Apra Mattoo1,2, Jeffrey M Stern1, Ian Jaffe1, Karen Khalil1, Imad Aljabban1, Adam Griesemer1, Robert A Montgomery1, Edward Skolnik2,3, David Goldfarb2,4.
1Transplant Institute, NYU Langone Health, New York, NY, United States; 2Medicine, NYU Grossman School of Medicine, New York, NY, United States; 3Cell Biology, NYU Grossman School of Medicine, New York, NY, United States; 4Neuroscience and Physiology, NYU Grossman School of Medicine, New York, NY, United States
NYU Xenotransplant Research Group.
Introduction: In xenotransplantation, an important area of concern has been whether a pig kidney in a human body would fully recapitulate renal hormone production and response. One question is whether human anti-diuretic hormone (ADH), arginine-8-vasopressin (AVP) can activate the porcine vasopressin receptor. Another is whether renin secreted by a pig kidney can cleave human angiotensinogen, eventually leading to aldosterone release. As the AVP response and renin-angiotensin-aldosterone system (RAAS) are the main drivers of renal water and sodium handling, we sought to evaluate their function in a brain-dead human recipient who had undergone xenotransplantation with a genetically modified porcine kidney after bilateral native nephrectomy.
Methods: AVP and copeptin levels were measured to confirm brain death-induced central diabetes insipidus. Urine output, serum sodium levels, urine osmolality (Uosm), and their response to exogenous vasopressin infusion were monitored to assess the response of the porcine xenograft to AVP. Plasma renin concentration (PRC), plasma renin activity (PRA), and plasma aldosterone concentration (PAC) were measured in steady-state conditions and after a captopril challenge using immunoassays.
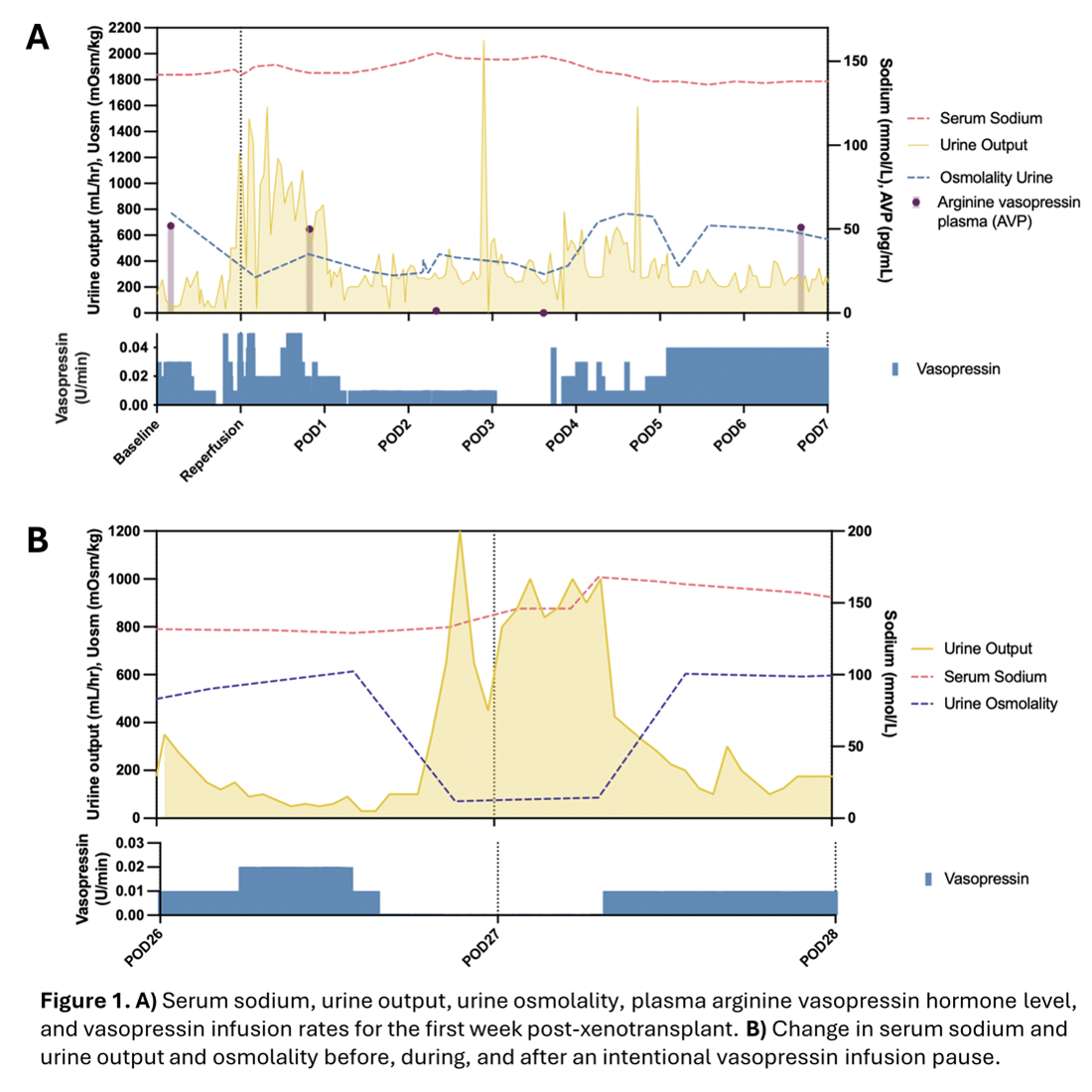
Results: Initiation of vasopressin infusion per standard brain death management protocols led to the normalization of urine output and serum sodium levels, and increased Uosm by the end of the first post-operative week (Figure 1A). The decedent’s copeptin levels were near undetectable throughout. Vasopressin infusion was discontinued on POD27 to establish the xenograft's responsiveness to AVP. We observed a significant increase in urine output from 100 ml/hr to >1000 ml/hr, accompanied by a rise in serum sodium from <130 meq/L to >150 meq/L and a fall in Uosm to <100 mOsm/kg (Figure 1B). These findings reversed upon resumption of the vasopressin infusion. PRA was 11.2 ng/mL/hr when measured pre-transplant and decreased to 0.6 ng/mL/hr on POD2 (Figure 2A). The pre-transplant PAC was 11.9 ng/dL and decreased to 3.8 ng/dL by POD5. PRA and PAC remained low or undetectable through the study's end. An adrenocorticotrophic hormone stimulation test excluded the possibility of adrenal insufficiency. PRC measured by ELISA in the donor pig’s serum was 207.8 pg/mL. However, pig renin was not measurable in any of the post-transplant decedent sera. The low pig PRC, PRA, and PAC may be attributed to the volume-expanded state of the decedent during the study. A captopril challenge, performed to stimulate renin release from the xenograft, resulted in a decrease in PAC from 4.8 ng/dL to undetectable and an increase in PRA from undetectable to 0.1 ng/mL/hr (Figure 2B).
Conclusion: A pig-to-human xenograft appears to appropriately respond to vasopressin and maintain water balance. There may also be partial preservation of RAAS signaling with some aldosterone production responsive to pig renin-mediated angiotensin production.
Funding for this study was provided by the United Therapeutics Corporation.
[1] xenotransplantation
[2] renin
[3] angiotensin
[4] aldosterone
[5] renin-angiotensin system
[6] renal transplantation
