Can blood products cause antibody-mediated xenograft rejection? Detection and removal of anti-pig xenoantibodies in human blood products
Massimo Mangiola1,2, Timothy Hilbert2, Amy Dandro3, Ana Fazio-Kroll3, Jacqueline Kim1, Karen Khalil1, Ian Jaffe1, Imad Aljabban1, Jeffrey M Stern1, Robert A Montgomery1, Adam Griesemer1.
1Transplant Institute, NYU Langone Health, New York, NY, United States; 2Pathology, NYU Grossman School of Medicine, New York, NY, United States; 3Revivicor, Blacksburg, VA, United States
Introduction: Preformed xenoreactive antibodies against non-alpha-Gal porcine epitopes can threaten xenograft viability. However, little is known about the presence of donor reactive xenoantibodies (xAb) in blood bank-issued products and the capacity of these products to passively transfer xenoantibodies.
Methods: Multiple samples of blood bank products (cryoprecipitate (CRYO), fresh frozen plasma (FFP), and human packed red blood cells (PRBC) both pre- and post-washing using a Haemonetics automated cell processing system) were obtained from our institution’s blood bank for intended transfusion in a decedent xenograft kidney recipient. These samples were tested for the presence of IgG and IgM xAb against alpha-Gal KO pig peripheral blood mononuclear cells (PBMC) by flow cytometry crossmatch (FCXM).
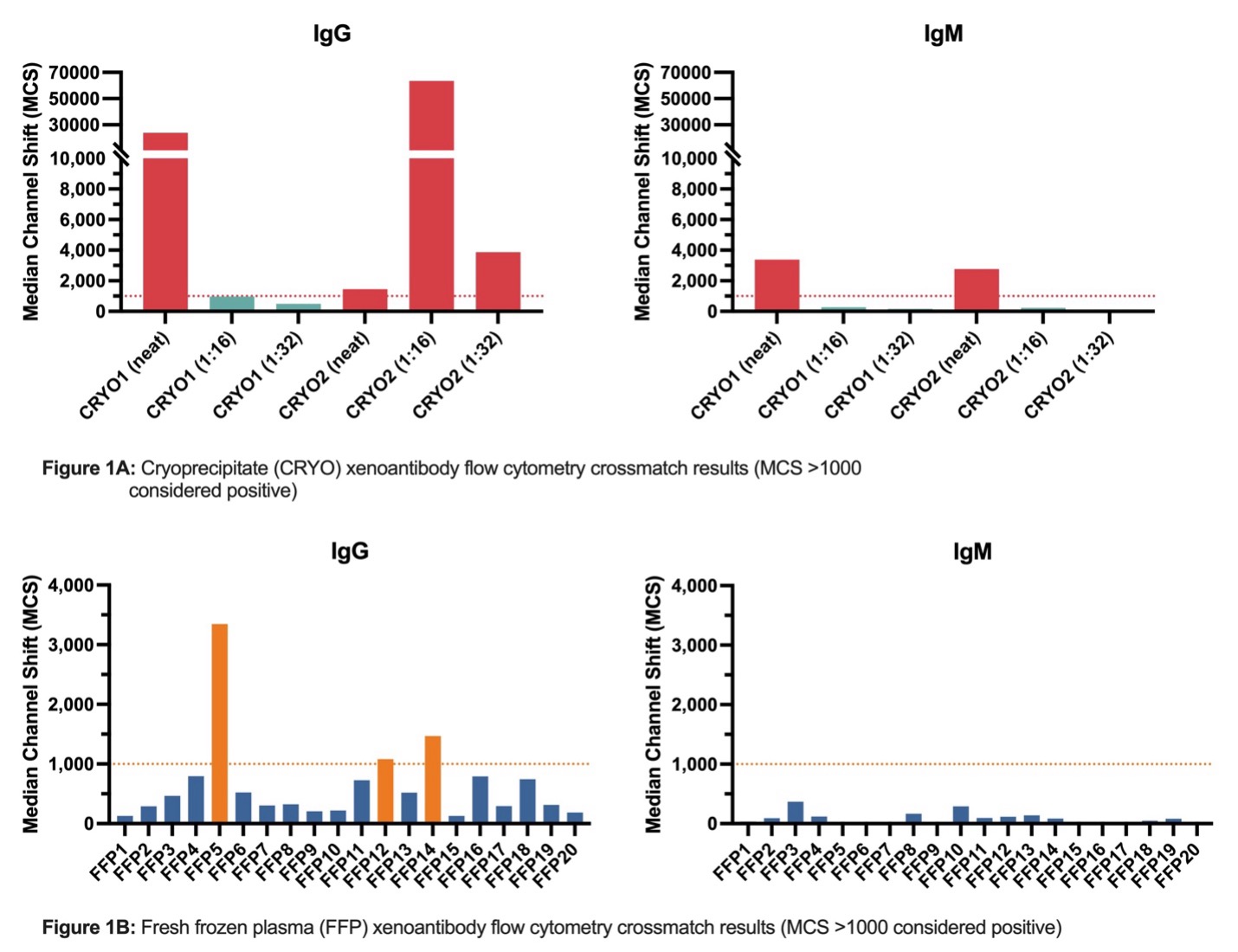
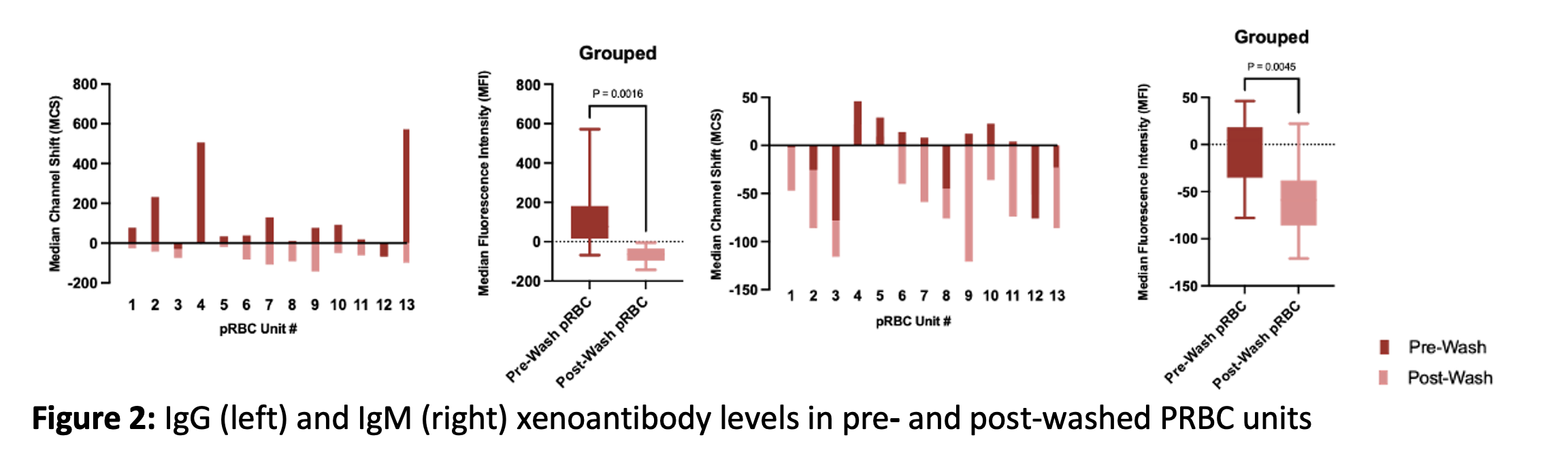
Results: CRYO and FFP units were found to have variable levels of xAb, some of which contained titers so high that they artificially lowered the observed amount of xAb by inhibiting the assay’s secondary IgG (the “prozone effect”). Other units were not predicted to cause complement activation given that the FCXM median channel shift (MCS) at 1:16 dilution fell below the positive threshold of 1000. (Figure 1A-1B) Similarly, PRBCs were found to have detectable levels of IgG and IgM non-alpha Gal xenoreactive antibodies at 1:16 dilution, which were significantly (IgG p <0.0016, IgM p <0.0045) reduced following washing (Figure 2).
Conclusion: Standard blood bank-issued products contain varying levels of xenoreactive IgG and IgM antibodies against non-alpha-Gal epitopes, which warrants prospective testing of products prior to transfusion in a xenograft recipient to prevent passive introduction of xAb. The washing process significantly reduces xAb levels and should be considered when transfusing PRBCs in clinical xenotransplantation.
Funding for this study was provided by the United Therapeutics Corporation.
[1] xenotransplantation
[2] antibody
[3] transfusion medicine
[4] graft rejection
[5] tissue crossmatching
