Efficacy of 12-week pegcetacoplan in kidney transplant recipients with recurrent C3 glomerulopathy (C3G) or immune complex membranoproliferative glomerulonephritis (IC-MPGN)
Andrew Bomback1, Erica Daina2, John Kanellis3, David Kavanagh4, Matthew C Pickering5, Gere Sunder-Plassmann6, Patrick Walker7, Zhongshen Wang8, Zurish Ahmad8, Fadi Fakhouri9.
1Columbia University Irving Medical Center, New York, NY, United States; 2Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Bergamo, Italy; 3Monash Medical Centre, Clayton, Australia; 4National Renal Complement Therapeutics Centre, Newcastle University, Newcastle upon Tyne, United Kingdom; 5Imperial College, London, United Kingdom; 6Medical University of Vienna, Vienna, Austria; 7Arkana Laboratories, Little Rock, AR, United States; 8Apellis Pharmaceuticals, Inc., Waltham, MA, United States; 9Lausanne University Hospital, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
Introduction: Pegcetacoplan, a targeted C3 inhibitor, may prevent C3G or IC-MPGN progression. NOBLE (NCT04572854) is the first prospective randomized controlled trial of pegcetacoplan vs standard of care (SOC) in kidney transplant recipients with primary C3G or IC-MPGN recurrence.
Method: Adult patients were randomized 3:1 to subcutaneous pegcetacoplan 1080 mg twice weekly plus SOC (n=10) or SOC only (n=3). The primary endpoint was a reduction in renal biopsy C3c staining (≥2 orders of magnitude [OOM]) from baseline to week 12. Additional week-12 endpoints included changes in estimated glomerular filtration rate (eGFR), urine protein-to-creatinine ratio (uPCR), C3G activity score, serum C3, and serum sC5b-9.
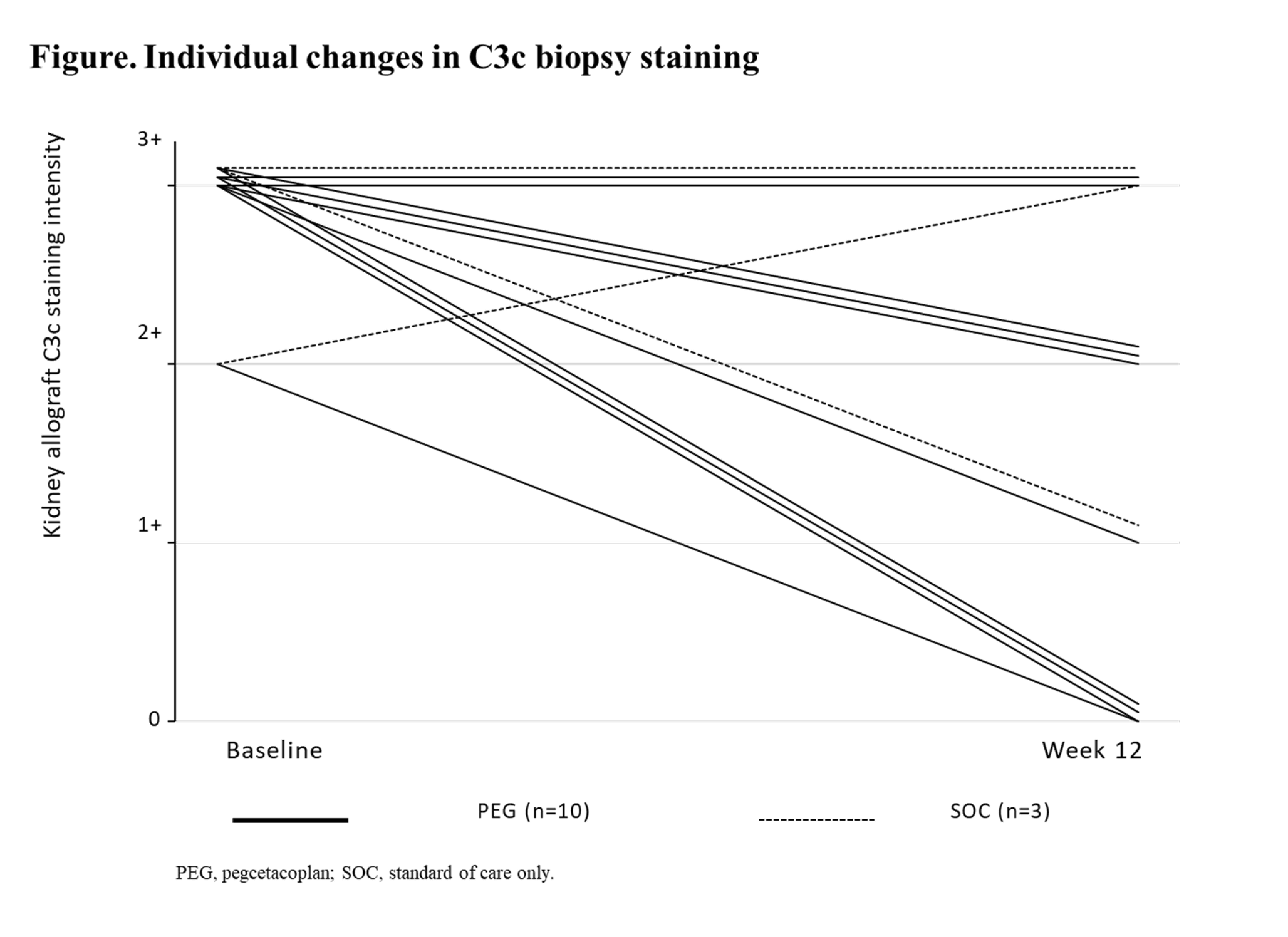
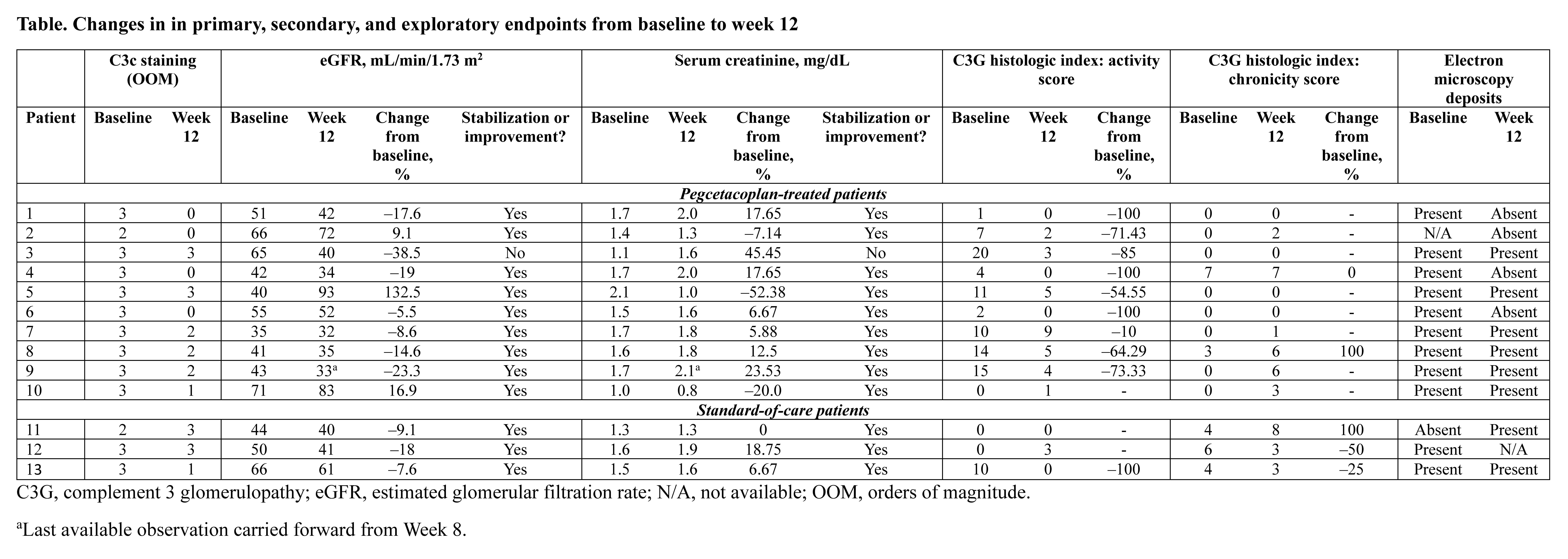
Results: 9 (69.2%) patients had C3G and 4 (30.8%) had IC-MPGN. At week 12, 5 (50%) pegcetacoplan-treated patients had ≥2 OOM reduction in C3c staining (4/5 had 0 intensity and cleared electron microscopy deposits); 8 (80%) pegcetacoplan-treated patients had ≥1 OOM reduction (Figure). 9 (90%) pegcetacoplan-treated patients had reduced C3G activity score at week 12. In a subgroup of patients with ≥1000 mg/g uPCR at baseline, uPCR decreased with pegcetacoplan (–39.2%) at week 12. eGFR remained stable, serum C3 increased, and sC5b-9 decreased with pegcetacoplan treatment (Table). There were no discontinuations or deaths due to treatment-emergent adverse events.
Conclusion: As early as week 12, pegcetacoplan reduced C3c staining and proteinuria with stable eGFR, targeted the pathophysiology of C3 dysregulation, and was well tolerated in kidney transplant recipients with recurrent C3G or IC-MPGN. A significant decrease of C3c deposits in the kidneys combined with a sustained increase of serum C3 and a reduction of plasma sC5b9 levels is consistent with the decreased breakdown of C3, which has not been previously demonstrated with proximal alternative pathway or terminal C5 complement blockade.
This study was funded by Apellis Pharmaceuticals, Inc., and Swedish Orphan Biovitrum AB. Medical writing support was provided by Kay Square Scientific and funded by Apellis. .
