Covid-19 hospitalization among liver transplant recipients: Multicenter study of clinical outcomes in the United States
Robert L Gottlieb1,2,3, Aastha Chandak4, Linda Chen5, Heng Jiang4, Kyung min Kwon5, Catherine Frenette5, Essy Mozaffari5.
1Baylor University Medical Center, Dallas, TX, United States; 2Baylor Scott & White Heart and Vascular Hospital, Dallas, TX, United States; 3Baylor Scott & White The Heart Hospital,, Plano, TX, United States; 4Certara, New York, NY, United States; 5Gilead Sciences, Foster City, CA, United States
Background: AASLD issued an FAQ in May 2022 highlighting the impact of COVID-19 on liver transplant recipients. The objective of this study is to examine the characteristics, treatments, and outcomes of liver transplant recipients hospitalized with COVID- 19.
Methods: We identified a heterogenous cohort of liver transplant recipients who underwent liver transplant procedures, experienced liver transplant complications, or had liver transplant-related visit between Jan 2019-Mar 2022 in the Premier Healthcare US Database. Among these patients, we identified subsequent COVID-19 hospitalization between Apr 2020-Mar 2022. Demographics, comorbidities, COVID-19 severity defined as oxygenation requirements during hospitalization, COVID-19 medications, immunosuppression medications, and inpatient all-cause mortality were examined.
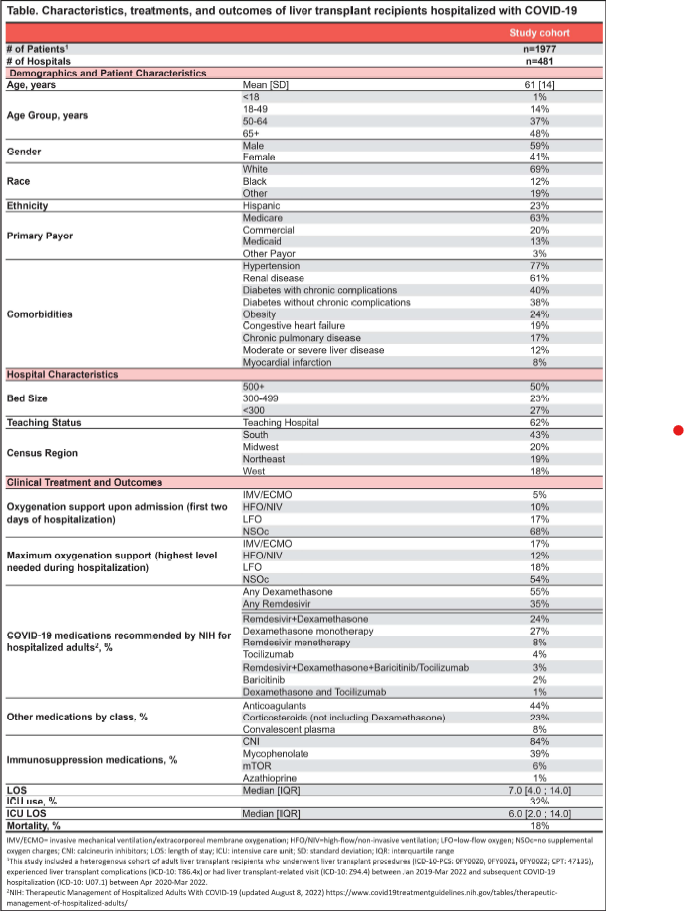
Results: 1,977 liver transplant recipients were hospitalized for COVID-19 in 481 hospitals of varying size, type, and location (Table). The average age was 61 years (S0=14), 59% male, 69% white, and 23% Hispanic. Key recorded comorbidities included: 77% hypertension, 61 % renal disease, 40% diabetes with chronic complications, and 38% diabetes without chronic complications.
On admission, 5% received invasive mechanical ventilation (IMV/ECMO), 10% high-flow/non-invasive ventilation (HFO/NIV), and 17% low-flow oxygen (LFO). During the entire hospitalization, 17% received IMV/ECMO, 12% HFO/NIV, 18% LFO as maximal oxygen support.
Key COVID-19 medications documented were: 55% dexamethasone, 35% remdesivir, and 23% corticosteroids other than dexamethasone. Additionally, calcineurin inhibitors (CN I) were documented for majority of the patients (84%) and mycophenolate for 39%. Few were reported to receive mTOR (6%) or azathioprine (1 %).
32% were admitted to ICU during hospital stay and inpatient all-cause mortality rate was 18%.
Conclusion: This study captures COVID-19 hospitalizations among liver transplant recipients over two years of the pandemic, across geographically representative institutions in the US. Inpatient all-cause mortality rate in this high-risk population is higher compared to the general population (16%) per our previous analyses of COVID-19 hospitalizations. Although corticosteroids are prescribed for COVID-19 with hypoxemia or for transplant, dexamethasone is not typically used for liver transplant immunosuppression; its use in the majority confirms a principal. More recent data will be presented at the conference upon acceptance.
[1] COVID-19
[2] Liver transplantation
[3] Outcomes
