High PD-L1 expression on Toll-Like-Receptor-stimulated-Bregs as keystone of regulatory function in PD-L1-PD1 interactions with CD4+T cells
Minxue Liao1,2,3, Kang Mi Lee1, Katsuhiro Tomofuji1,2, Kevin Deng1, Gaoping Zhao4, Shaoping Deng4, Lei Ji1,2,3, James F. Markmann3.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States; 2Department of Surgery, Massachusetts General Hospital, Boston, MA, United States; 3Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States; 4School of Medicine, University of Electronic Science and Technology of China, Chengdu, People's Republic of China
Markmann lab.
Introduction: We aim to elucidate the crucial role of PDL1-PD1 signaling in the modulation of anti-graft responses by TLR-Bregs.
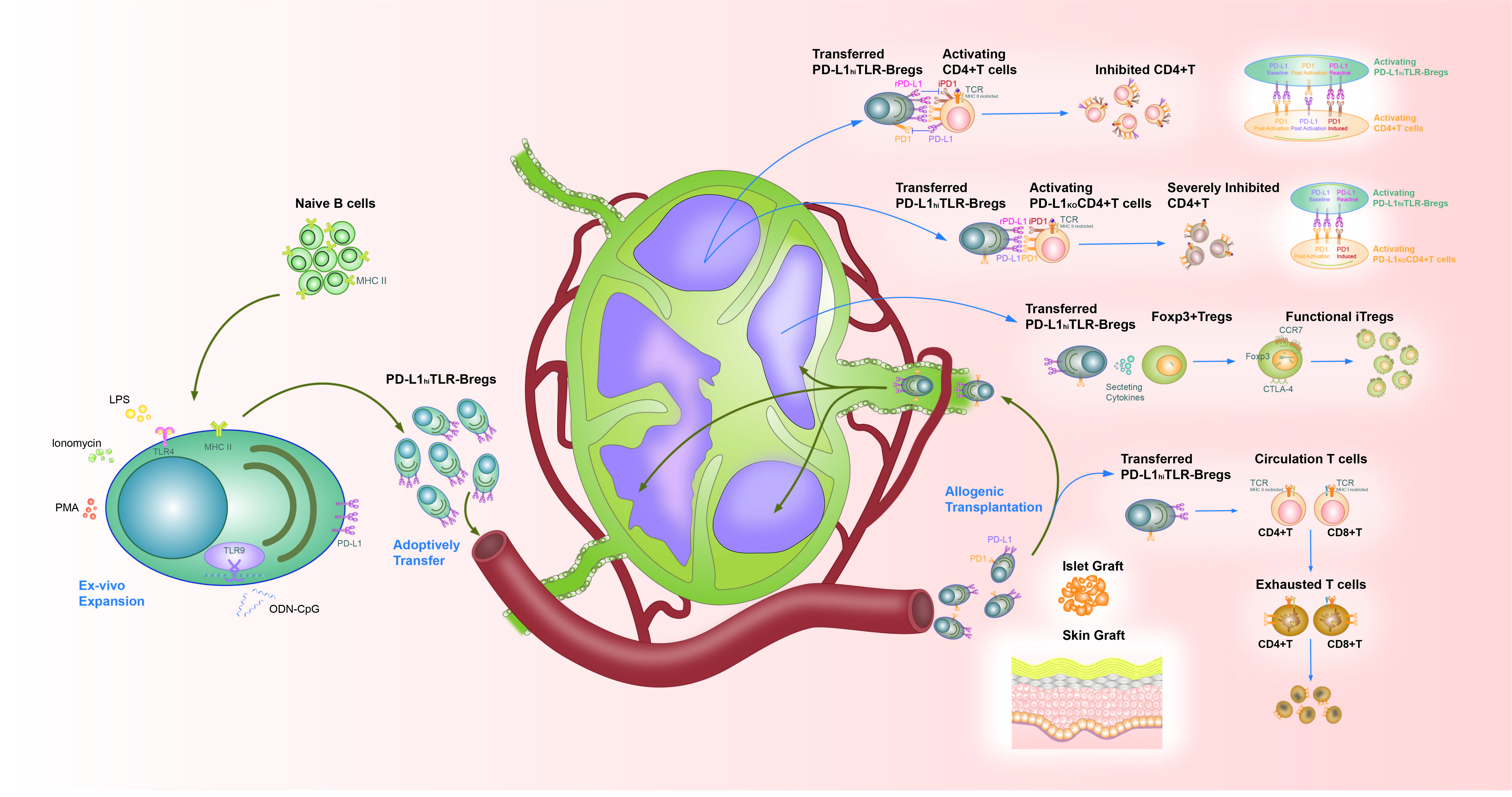
Method: We expanded Bregs ex-vivo from the splenic B cells of wild-type C57BL/6 (WT) or PDL1 deficient (KO) mice. TLR-Bregs were generated via TLR9 and TLR4 signaling, while CpG-Bregs (TLR9 stimulation) served as controls. We compared these putative Bregs to evaluate their impact on graft survival, modulation of T cell exhaustion, Treg induction, and regulation of activated CD4+T cells.
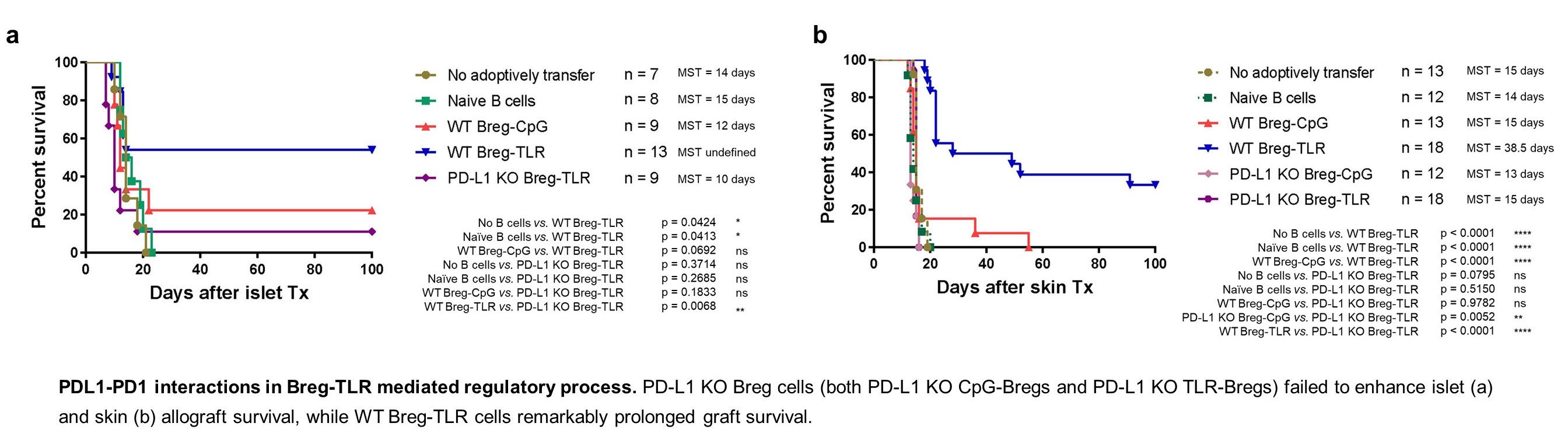
Results: WT TLR-Bregs, but not PDL1KOTLR-Bregs, significantly extended islet and skin graft survival in WT B6 recipients. Phenotypic analysis revealed all Bregs were in the transitional stage and manifested rare PDL1 but higher PD1 expression in KOTLR-Bregs than WT. KOTLR-Bregs were less effective in modulating higher PD1 and lower PDL1 expression (exhaustion) in recipient circulating T cells. WT TLR-Bregs induced a greater proportion of Foxp3hiCCR7hiCTLA4hiTregs in the draining lymph node, while KOBregs generated fewer Tregs with less functional markers. Notably, PDL1 transfer was observed in-vivo and in-vitro between activating TLR-Bregs and CD4+T, affecting the expression profiles in KO cells. Uniquely, WT TLR-Bregs suppressed the PD1-expressing activating WT CD4+T in-vivo and in-vitro in a dose-dependent manner. Conversely, WT CpG-Bregs and TLR-Bregs dose-dependently suppress activating KOCD4+T. Specifically, WT TLR-Bregs showed further improved graft survival, enhanced inhibitory function, and increased Breg survival with KOCD4+T than WT CD4+T. We then discovered that activating WT CD4+T (especially engaged TLR-Bregs) highly expressed PDL1, while activating Bregs were PD1 expressing. Post in-vivo and in-vitro contact with PDL1hiCD4+T, KOBregs manifest reduced cell survival, less PDL1 expression, and lower PD1+cell proportion but higher PD1 protein than WT TLR-Bregs. Moreover, PDL1 expression on WT TLR-Bregs further increased after suppressing PDL1hiCD4+T, and WT TLR-Bregs up-regulated PD1 protein on activating WT PDL1hiCD4+T. Dynamic expression changes in PD1 and PDL1 on Bregs and CD4+T cells indicated activated PDL1hiCD4+T counteract PD1+Bregs; while WT Breg-TLR, but not KOBregs, secure their regulatory capacity by up-regulating self PDL1 and PD1 on CD4+T cells.
Conclusion: High PDL1 expression on TLR-Bregs is foundational to their regulatory function. PDL1 deficiency in TLR-Bregs impairs their ability to suppress CD4+T cell activation, promotes T cell exhaustion, induces functional iTregs, and prevents the antagonistic effects of activated PDL1hiCD4+T cells. Conversely, PDL1 deficiency on CD4+T enhances Bregs' regulatory capacity. This study underscores the therapeutic potential of targeting the PDL1-PD1 pathway on Bregs in enhancing allograft tolerance.
This abstract is accepted by ATC 2024 as oral presentation.
[1] Regulatory B cells
[2] Graft tolerance
[3] Tregs
[4] T cell exhaustion
