Impact of donor-recipient body surface area mismatch on kidney graft survival and function
Tanawat Yothinarak1, Napun Suttharattanapong2,3, Surasak Kantachuvesiri2,3, Rungthiwa Kitpermkiat2,3, Adisorn Pathumarak2,3, Sitta Jiriyasin 4, Kun Sirisopana 3,5, Sarinya Boongird2,3.
1Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Division of Nephrology, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 3Excellence Center of Organ Transplantation, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 4Bangpakok 9 international hospital, Bangkok, Thailand; 5Division of Urology, Department of Surgery, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Introduction: Discrepancies between donor and recipient body size can lead to glomerular hyperfiltration or underfiltration, potentially compromising long-term graft survival and function. This study investigates the impact of kidney size mismatch, assessed by the body surface area (BSA) ratio, on death-censored graft failure and estimated glomerular filtration rates (eGFR) in deceased donor kidney transplant (DDKT) recipients.
Method: We retrospectively analyzed adult DDKT recipients from Ramathibodi Hospital between January 2010 and December 2022. Recipients were categorized into three groups based on the body surface area donor-to-recipient (BSA D:R) ratio. Cut-offs were defined using ±1 standard deviation (SD) from the mean BSA D:R of the entire cohort, creating small-donor (BSA D:R < 0.90), match-donor (BSA D:R 0.90–1.25), and large-donor (BSA D:R > 1.25) groups. Kaplan-Meier analysis and the log-rank test was used to evaluate death-censored graft failure among the groups. Cox-proportional hazard models were used to identify factors affecting graft failure. We used mixed-effects linear regression to analyze the eGFR trajectories within and across each category over the follow-up period.
Results: of 1,094 DDKT recipients, 185 (17%) were classified as small donors, 743 (67%) as matched donors, and 166 (16%) as large donors. Baseline graft quality was comparable across groups. Over a median (IQR) follow-up of 4.7 (5.1) years, 121 patients experienced death-censored graft failure. Kaplan-Meier analysis with log-rank test (p = 0.007) demonstrated a significant difference in graft failure among recipient groups based on BSA D:R ratio.
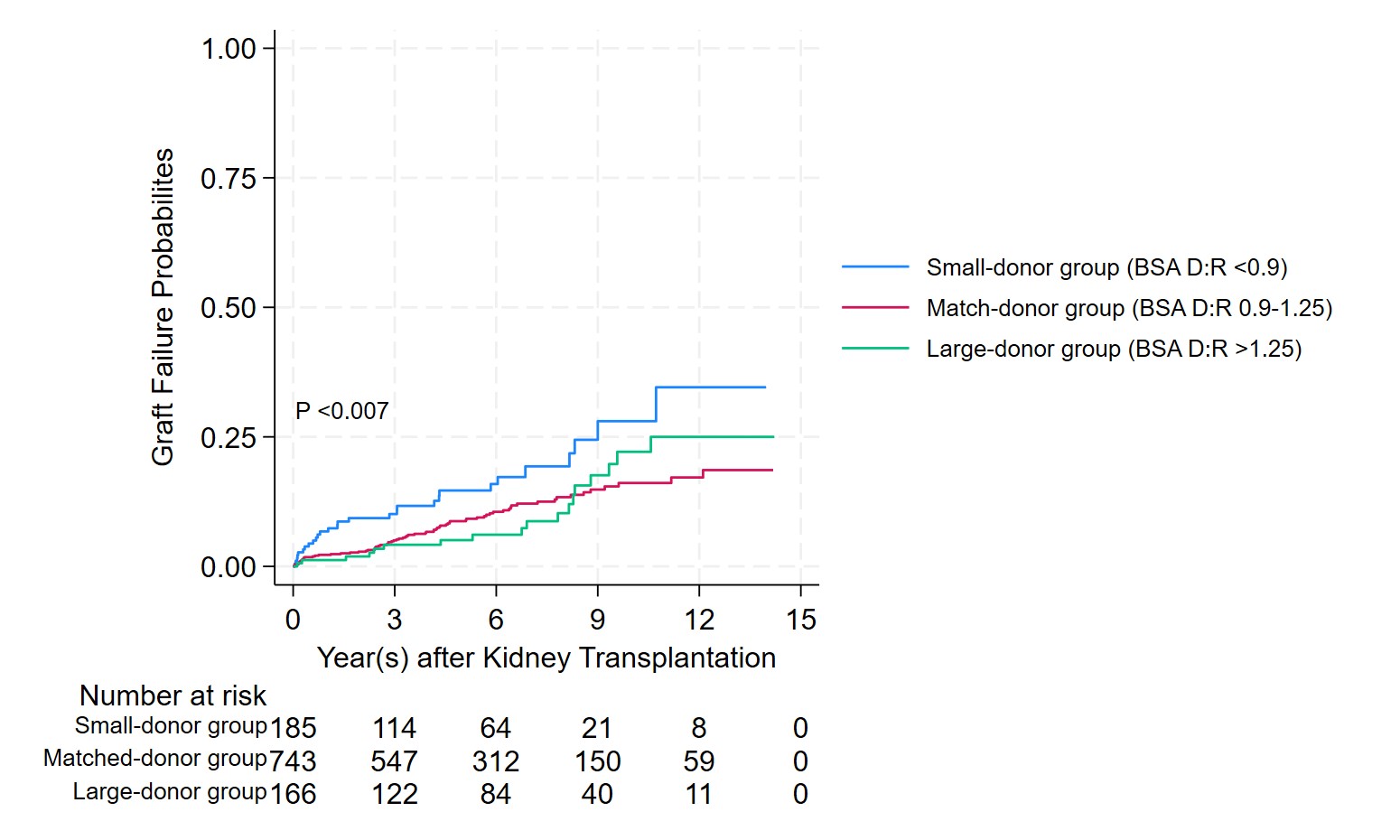
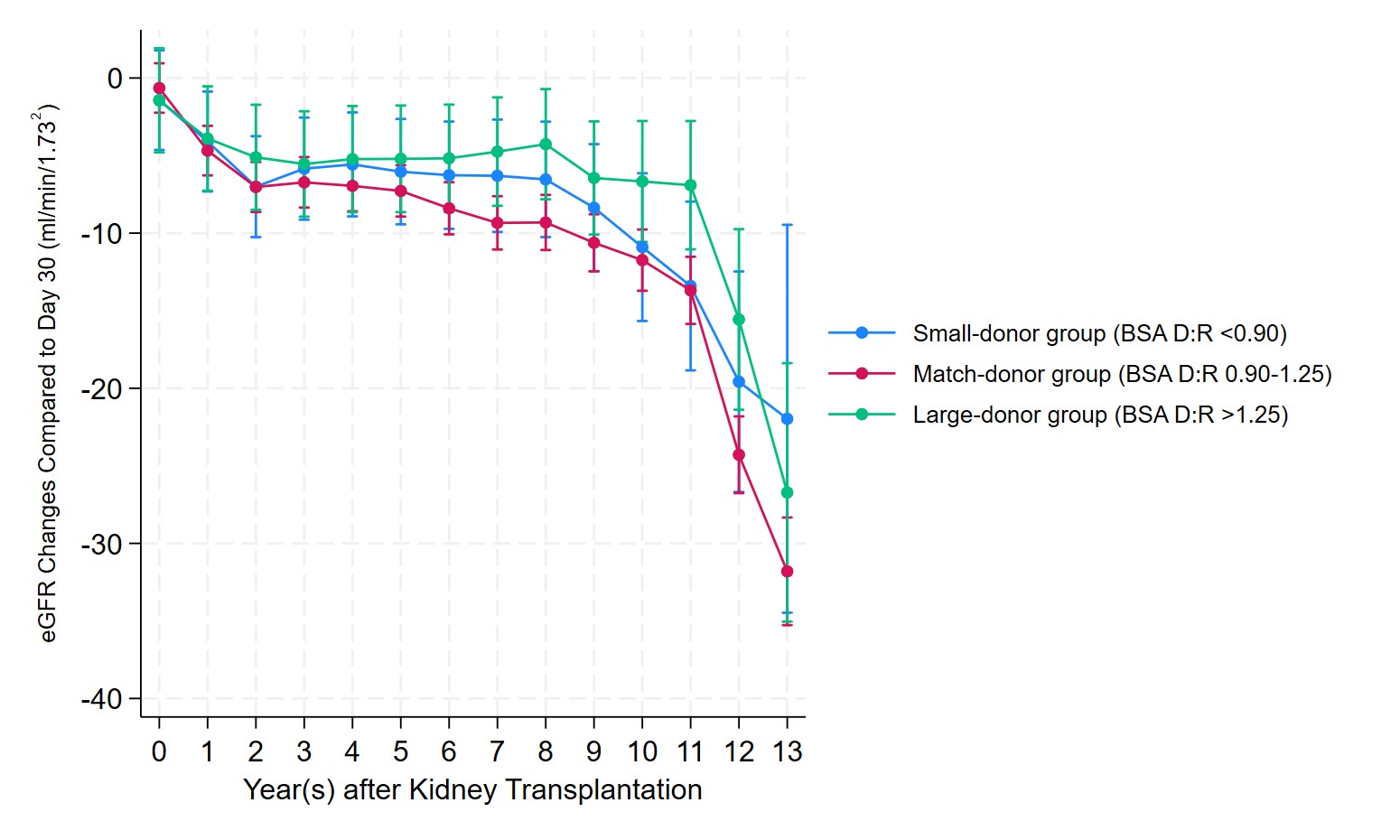
After adjusting for donor and recipient age, expanded criteria donor status, acute kidney injury in the donor, and HLA mismatch, recipients in the small-donor group had a 2.03-fold increased risk of graft failure compared to the match-donor groups (adjusted HR: 2.03, 95% CI: 1.32–3.14, p <0.01). No significant diferrence in graft failure risk was observed between the large-donor and match-donor groups (adjusted HR: 0.81, 95%CI: 0.40–1.39, p = 0.45). Within each group, after adjusting for baseline eGFR at 30 days post-transplant, the match-donor and small-donor groups exhibited similar eGFR trajectories over time. In contrast, the large-donor group displayed a significantly slower decline in eGFR compared to the small-donor group (mean difference: 2.2 ml/min/1.73 m², p = 0.04) (Figure 2).
Conclusions: This study demonstrates that recipients with small donor kidneys experience a significantly increased risk of death-censored graft failure compared to those receiving match-sized or large donor kidneys. This finding highlights the importance of considering kidney size mismatch as a factor influencing long-term graft function following DDKT.