Standardizing usability testing for medical devices intended for intraoperative use during kidney transplantation
Jon Freise1, Thomas Sorrentino1, Kyle Hartlet2, Darja Wendel2, Marc Melcher2, James Gardner1, Keith Hansen1.
1Department of Surgery, University of California, San Francisco (UCSF), San Francisco, CA, United States; 2Department of Surgery, Stanford University, Palo Alto, CA, United States
Introduction: Medical device development requires usability testing to ensure safety for users and patients. Evaluating user input informs device design which reduces costs and improves users’ experiences. Given the costs of device development, there is a demand for standardized, cost-effective models and protocols for usability testing. In this study, we developed and validated a protocol and simulation model for standardized testing of medical devices intended for intraoperative use during kidney transplants.
Methods: Our team developed a task analysis and use-related risk analysis (URRA) for a device intended for intraoperative cooling during kidney transplants. The URRA identifies potential use errors and guides usability protocol evaluations with a focus on critical tasks that may cause serious harm. Materials and supplies, estimated ~ $1000 USD, were obtained with surgeon input to simulate the vascular anastomoses of a kidney transplant. Supplies included a mock operating room table, lighting, sutures, vascular clamps and instruments, along with porcine kidney and aorta specimens (Figure 1). The iliac fossa was replicated with a previously validated 3-dimensional model1 that was modified into a soft tissue-mimicking silicone mold (Figure 1). A usability protocol was developed to evaluate use of the device (Figure 2). Transplant surgeons were recruited for the study.
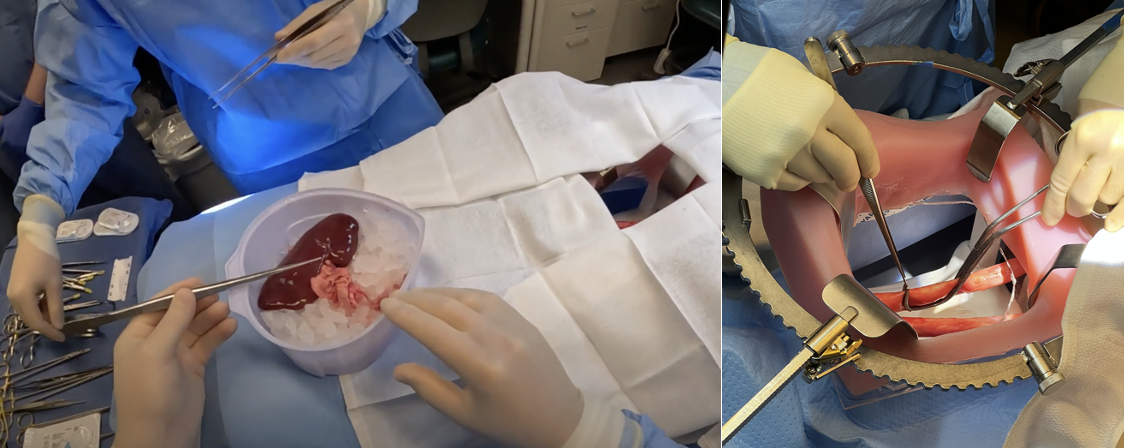
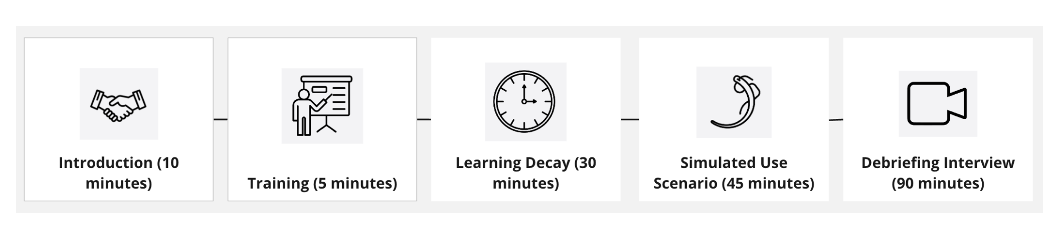
Result: Seven surgeons were recruited for usability testing over 6 sessions. Each 3 hour session included a training, a learning decay period, and a simulated use scenario with debriefing interview (Figure 2). Each session included an end user (transplant surgeon), an observer, a note-taker, and proxy assistant surgeon. Surgeons were tasked with performing a vascular anastomosis with the porcine kidney and prototype device using the porcine kidney artery (donor) and aorta (recipient) vessels as part of this study. Sessions were recorded and reviewed to identify themes and insights related to product features and workflow processes. All seven surgeons stated that the simulated model was an adequate simulation of the vascular anastomosis during a kidney transplant procedure. Moreover, they noted the simulation model enabled them to replicate any potential use errors that might arise in a comparable cadaveric model or actual transplant.
Conclusion: As healthcare demands innovation for more complex challenges, standardization and accessibility is needed around product development, including usability testing. In this study, we validated a successful model and usability protocol that can be used as guidance for development of devices intended for intraoperative use during kidney transplantation worldwide.
References:
1. Jake Claflin, Seth A. Waits, Three Dimensionally Printed Interactive Training Model for Kidney Transplantation, Journal of Surgical Education, Volume 77, Issue 5, 2020, Pages 1013-1017, ISSN 1931-7204
Stanford Catalyst.
[1] Usability
[2] Medical Device
[3] Innovation
[4] Kidney Transplant
[5] Simulation
