Clinical utility of 1:16 serum dilution as a predictor of response to therapeutic plasma exchange for HLA antibody-mediated rejection treatment and overall survival in lung transplant recipients: A two center study
Mohamed Elrefaei1, Tathagat Narula2, Francisco Alvarez2, Elizabeth A Godbey1, Gerard Criner3, Francis C Cordova3, Norihisa Shigemura, 4, Yoshiya Yoshiya Toyoda4, Olga Timofeeva5.
1Department of Laboratory Medicine and Pathology, Mayo Clinic, Jacksonville, FL, United States; 2Division of Lung Failure and Transplant, Mayo Clinic, Jacksonville, FL, United States; 3Department of Thoracic Medicine and Surgery, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, United States; 4Department of Surgery, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, United States; 5Department of Laboratory Medicine and Pathology, MedStar Georgetown University Hospital, Washington DC, DC, United States
Background: Antibody-Mediated Rejection (AMR) due to HLA donor‐specific antibodies (DSA) is associated with poor outcomes in lung transplant recipients (LTR). Successful AMR treatment using therapeutic plasma exchange (TPE) improves clinical outcomes in LTR. The objective of this study was to assess the clinical utility of 1:16 serum dilution HLA antibody test results as a predictor of response to TPE for AMR treatment in LTR.
Methods: A retrospective analysis of 21 LTR diagnosed with AMR due to de novo HLA DSA (dnDSA) and successfully treated with TPE was performed at Mayo Clinic (n = 7) and Temple University Hospital (n = 14). HLA antibodies were detected by Luminex single antigen beads assay. Mean Fluorescence Intensity (MFI) levels were measured before the 1st and after the 5th TPE session using undiluted and 1:16 diluted sera. Statistical analysis was performed using IBM® SPSS® Statistics (v26; Armonk, NY).
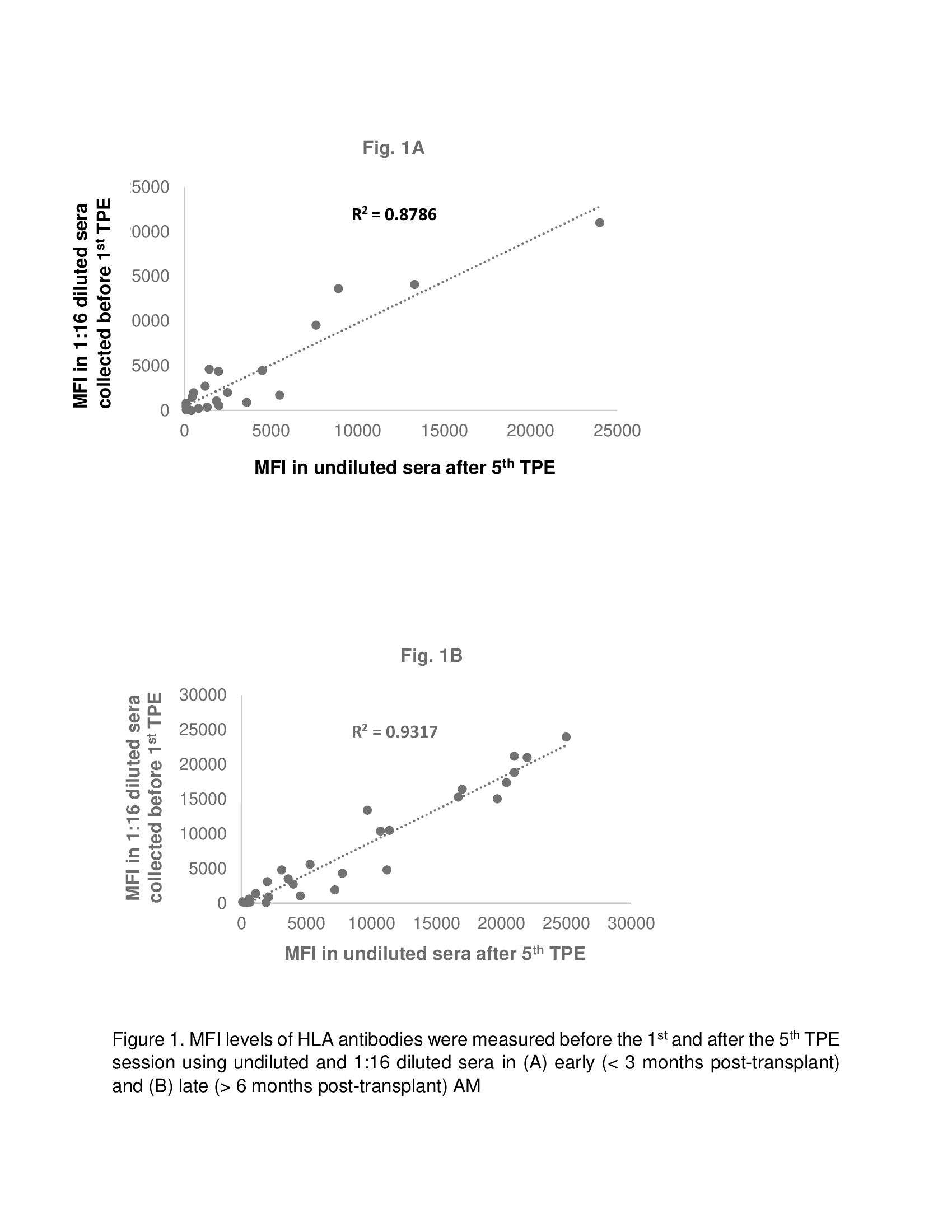
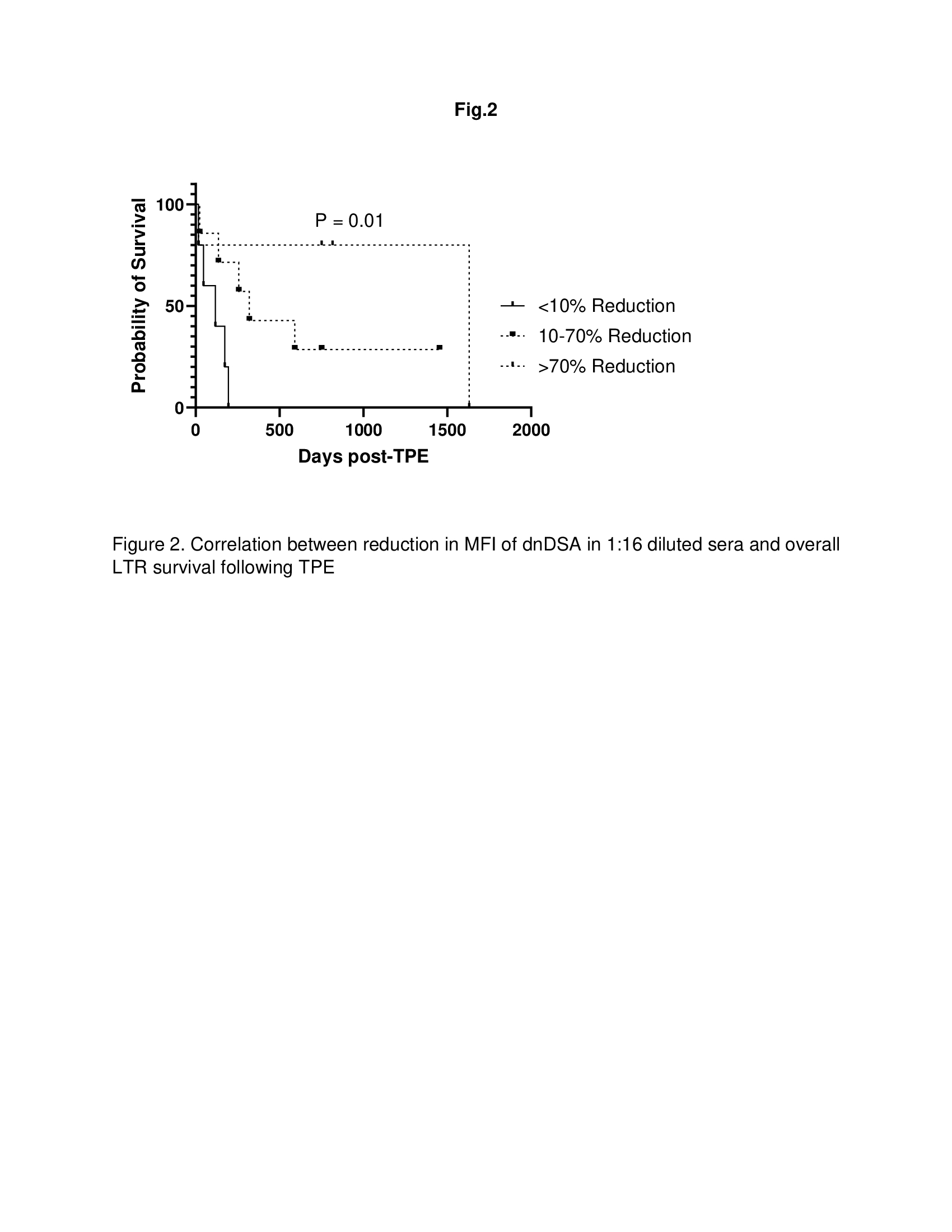
Results: Of 21 patients, 9 and 12 patients were diagnosed with early (< 3 months post-transplant) and late (6 months – 3 years post-transplant) AMR respectively. All patients had Class II dnDSA. In addition, 55% and 16% of LTR with early AMR and late AMR, respectively also had class I dnDSA. The MFI for all positive dnDSA in 1:16 diluted sera collected before 1st TPE demonstrated a significant correlation with MFI in undiluted sera collected 1 day after 5th TPE in both early (Fig. 1A; R2 = 0.8786) and late (Fig. 1B; R2 = 0.9045) AMR post-transplant. In addition, reduction in MFI of dnDSA in 1:16 diluted sera correlated with better overall LTR survival following TPE (Fig, 2; p = 0.01).
Conclusion: The MFI of 1:16 serum dilution before 1st TPE may be utilized as a surrogate to predict response to TPE for AMR treatment and overall survival in LTR.