Impacts of warm ischemia on myocardial protein phosphorylation after normothermic ex vivo reperfusion of hearts donated after circulatory death in a rat DCD model
Ling Gao1, Jeanette Villanueva 1,2, Aoife Doyle1, Yashutosh Joshi1,2,3, Sanjay Dutta1,2,3, Peter Macdonald1,2,3.
1Cardiac Transplantation Laboratory, Victor Chang Cardiac Research Institute, Sydney, Australia; 2St Vincent’s Clinical School, The University of New South Wales, Sydney, Australia; 3Department of Heart and Lung Transplantation, St Vincent’s Hospital, Sydney, Australia
Introduction: The global demand for hearts donated after circulatory death (DCD) for transplantation is increasing in an effort to expand the donor pool. Consequently, there is a need to better understand the impacts of warm ischemia on DCD heart functional recovery and molecular signaling. This is critical in the development of optimal preservation strategies to reduce the risk of post-transplant primary graft dysfunction. This study aimed to use an established rat DCD model (1) to investigate the impact of a 20 minute asystolic warm ischemic time (aWIT) on the phosphorylation status of key elements in signaling pathways where mitochondria are a common downstream target.
Methods: Male Wistar rats (340-420g, n=3-5 per group) were anesthetized, carotid artery cannulated for blood pressure monitoring, 500IU heparin injected, and the trachea ligated. Circulatory arrest was declared when pulse pressure zeroed. A 20 minute aWIT was observed before hearts were excised. Immediately after retrieval, all hearts received a flush of 100ml of ice-cold Celsior solution, followed by 1hr ex vivo reperfusion at 37°C with Krebs-Henseleit buffer for assessment of cardiac function recovery. Sham control hearts (subjected to no warm ischemia) were excised after heparin injection without tracheal ligation. Cardiac functional recovery was assessed by continuous measurement of cardiac output (aortic flow + coronary flow) during reperfusion. Left ventricle tissue samples were collected at the end of reperfusion for western blot of total and phosphorylated AMPK, Erk, Akt, Stat3 and Drp1. Levels of protein phosphorylation were presented as ratio to its total protein.
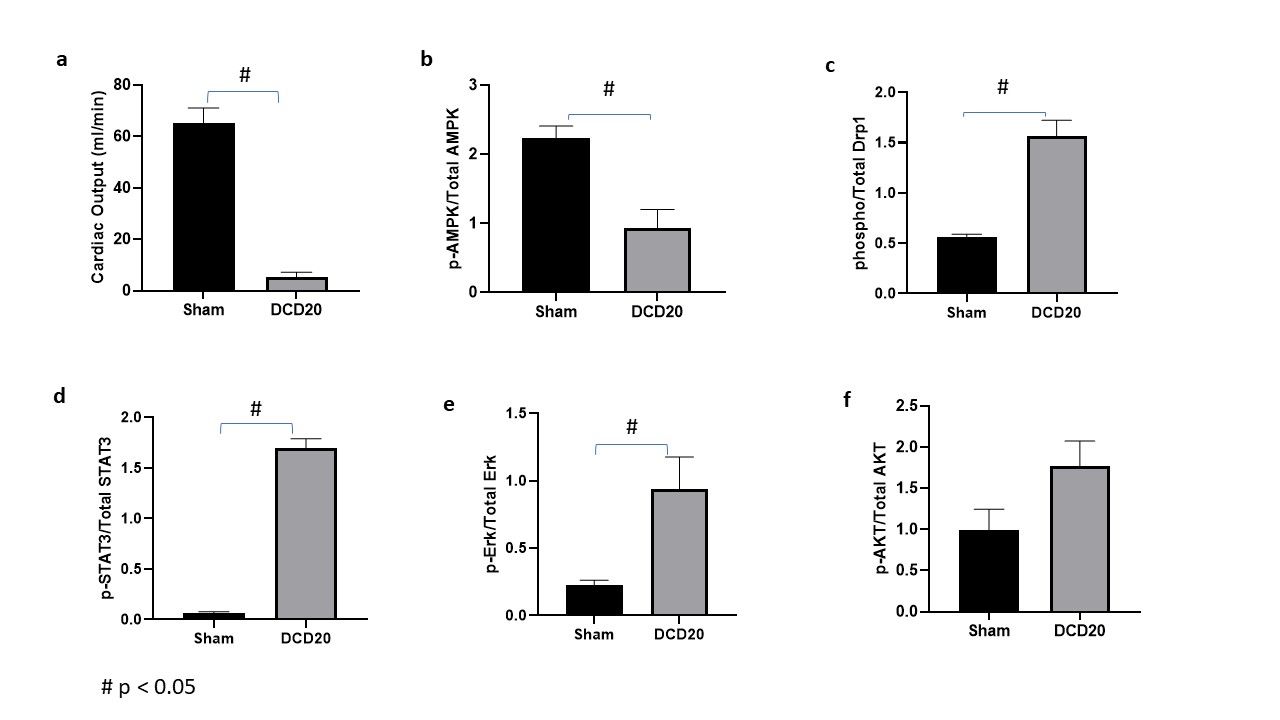
Results: Compared with Sham controls, DCD hearts had significantly reduced cardiac functional recovery after reperfusion. Cardiac output in hearts retrieved after 20 minute aWIT recovered only 8% to that of Sham controls (5.3±2.0 v 63±4.4 ml/min, p<0.05 Fig1a); Western blots showed significantly decreased AMPK phosphorylation in DCD hearts after 1hr reperfusion (Fig1b), while phosphorylation of Drp1S616 (Fig1c), Stat3 (Fig 1d) and Erk (Fig 1e) were significantly increased (p<0.05). Phosphorylation of Akt (Fig1f) was also increased in DCD hearts but not significantly so (p>0.05).
Conclusions: In our rat DCD model, hearts exposed to a 20min aWIT had a significant decrease of AMPK phosphorylation, accompanied by significantly increased phosphorylation of Drp1S616, a key promotor of mitochondrial fission activated through phosphorylation. Mutating the S616 phosphorylation site or pharmacologically inhibiting Drp1 activity has been reported to block mitochondrial permeability transition pore (mPTP) opening and prevent myocardial dysfunction in a mouse model (2). Our findings suggest the involvement of mitochondrial fission-mediated mPTP opening in DCD hearts during retrieval. Studies to explore this as a potential target of pharmacological conditioning against warm ischemia reperfusion injury in DCD hearts are well deserved.1). L. Gao et al. JHLT https://doi.org/10.1016/j.healun.2020.02.016. 2). H. Zhou et al. Redox Biology 15 (2018) 335–346.
National Health & Medical Research Council.
[1] Donation after circulatory death
[2] ex vivo reperfusion
[3] Warm ischemia
[4] Myocardia function
[5] Protein phosphorylation
