One year outcome of anti-T-lymphocyte globulin (ATLG) induction therapy in kidney transplantation: A single-center retrospective analysis
Kamal Kiran1.
1Nephrology , Medicover Hospital , Hyderabad, India
Introduction: Prevention of graft rejection is an important parameter in solid organ transplants. In renal transplants, anti-T lymphocyte globulin is commonly used as an induction agent. This data represents our experience using anti-T lymphocyte globulin in kidney transplants.
Materials and Methods: In a retrospective, single-center observational study, adult patients who underwent kidney transplants from 2020 to 2024 at our centre and had received ATLG as a part of induction were included in the study. The primary outcome measure was overall survival and graft function at 3, 6, 9, and 12 months. The primary outcome was assessed by development of graft rejection.
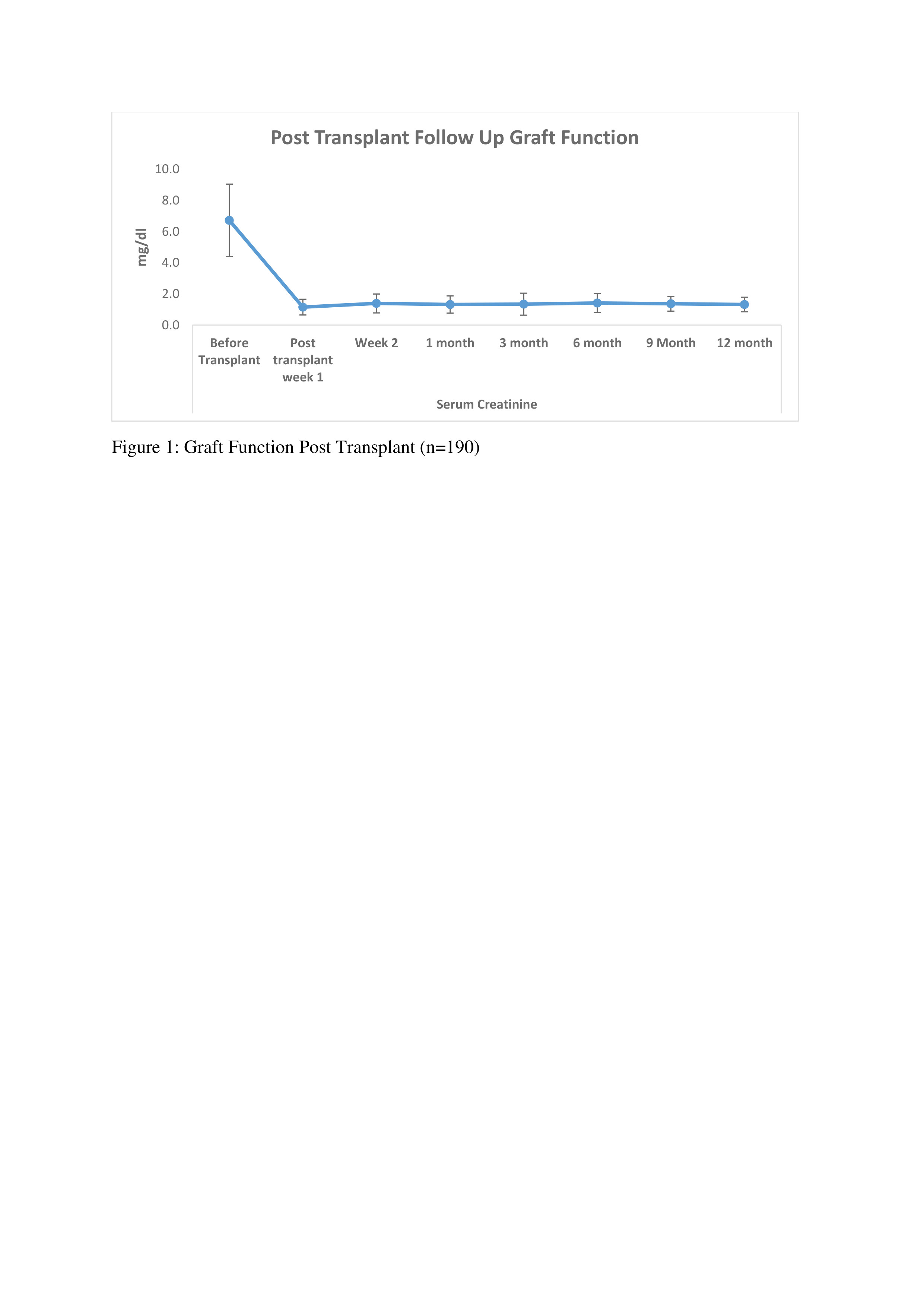
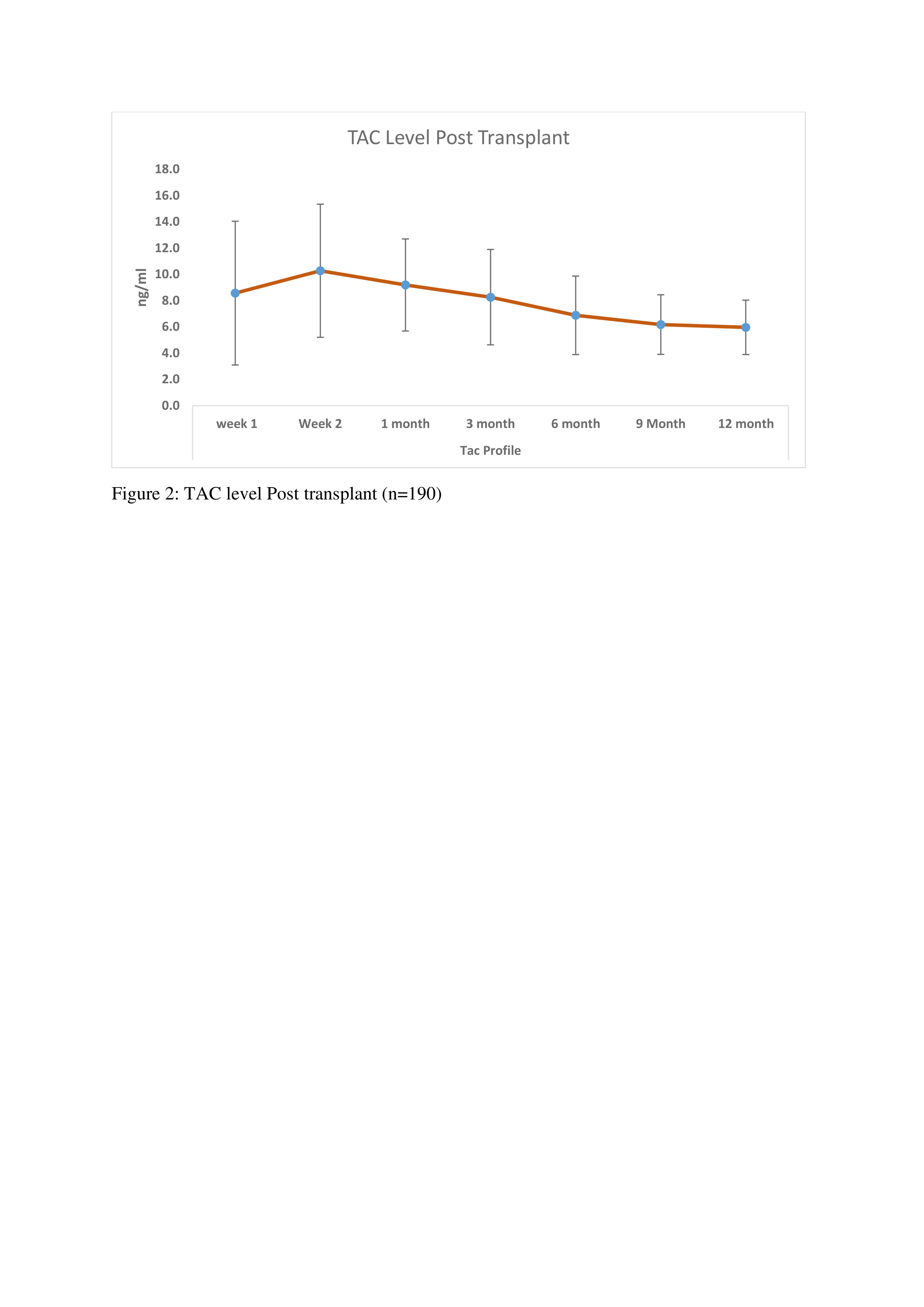
Results: In our retrospective analysis, 190 patients received ATLG as an induction agent on day of Transplant. The mean age of the patients was 40.23 ± 12.9 years. Out of 190, 149 (78.42%) were Male and 41 (21.57%) were Female. mean weight was 64.7 ± 12.6 kg. Regarding underlying kidney diseases, diabetic nephropathy (20%) and chronic glomerulonephritis (23.15%) were predominant. The mean cumulative dose of ATLG was 268.3 ± 89 mg, with a mean dose of 4.14 ± 1.4 mg/kg. Tacrolimus, mycophenolate sodium, and prednisolone were used as maintenance immunosuppressants. Patient survival rates were high at 186 (97.89%), with 4 (2.1%) patients dying due to septic shock and multiorgan dysfunction syndrome (MODS). Post-transplant graft function and tacrolimus levels were also evaluated at 3, 6, 9, &12 months, as shown in Figures 1 and 2. Rejection episodes included acute cellular rejection (12, 6.31%) and antibody-mediated rejection (4, 2.1%). Other complications observed in our study were acute tubular necrosis (11, 5.78%), acute tubular injury (4, 2.1%), pyelonephritis (2, 1.05%), and patchy necrosis of renal parenchyma (2, 1.05%). Disease recurrence occurred in 2, (1.05%) of cases, primarily IgA nephropathy. Additionally, re-admission post-transplantation was observed in 23, (12.1%) of cases.
Conclusion: In conclusion, this retrospective study shows that a low dose of ATLG (≈ 4 mg/kg) is effective in preventing graft rejection in kidney transplant patients. More studies are required to check the efficacy of ATLG in ABOi patients.