
Low-dose anti-T lymphocyte globulin (Grafalon) for kidney transplant induction immunosuppression. A pediatric cohort study
Martin Bitzan1,2, Marwa Mohamed1,3,4, Hazem Awad1, Haneen Yamin1, Shameer Habeeb1, Yazan Alkilani1, Abdalazeez Alghraizat 1, Gehad ElGhazali5,6, Waldo Concepcion1,2,3.
1Kidney Center of Excellence, Al Jalila Children's Hospital, Dubai, United Arab Emirates; 2Mohammed Bin Rashid University of Medicine and Health Sciences , Dubai, United Arab Emirates; 3Transplant Surgery, Al Jalila Children's Hospital, Dubai, United Arab Emirates; 4Surgical Oncology Research, Massachusetts General Hospital, Boston, MA, United States; 5Purehealth, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates; 6United Arab Emirates University, Al Ain, United Arab Emirates
Introduction Induction immunosuppression (IIS) employs lymphocyte-depleting and non-depleting antibodies (Ab), such as rabbit thymoglobulin (rATG) and IL-2 receptor-targeting basiliximab (BLX). T-cell suppression may cause CMV reactivation and malignancies. Optimal IS in pediatric transplantation is debated.
Aims of the study Evaluate low-dose anti-T lymphocyte globulin (ATLG, “Grafalon”) treatment for IIS in unselected pediatric KT recipients.
Outcomes of interest (1) Degree and duration of treatment-induced lymphocyte (ALC) depletion, and (2) rejection rates after ATLG vs BLX induction, incidence of infusion reactions, effects on neutrophil and platelet counts, rates of infection, and 1-year graft function post KT.
Method Retrospective single-center pediatric cohort study comprising all CKD5 patients who received a KT since 10/2018 (5.4 y; n=44 transplants; 34 (73%) from deceased and 10 from living-related donors). Standard protocol included ATLG 1.5 mg/kg on POD 0-2, IV induction and maintenance glucocorticoids (GC) and mycophenolate plus tacrolimus. In case of slow graft recovery, ATLG was extended to 5 doses. Few patients received BLX (POD 0/4) instead of ATLG. All were monitored for opportunistic viral infections. Data analysis is descriptive. Continuous variables are shown as arithmetic means ± SD or median and range. Student’s t and Fisher’s exact tests were used where indicated.
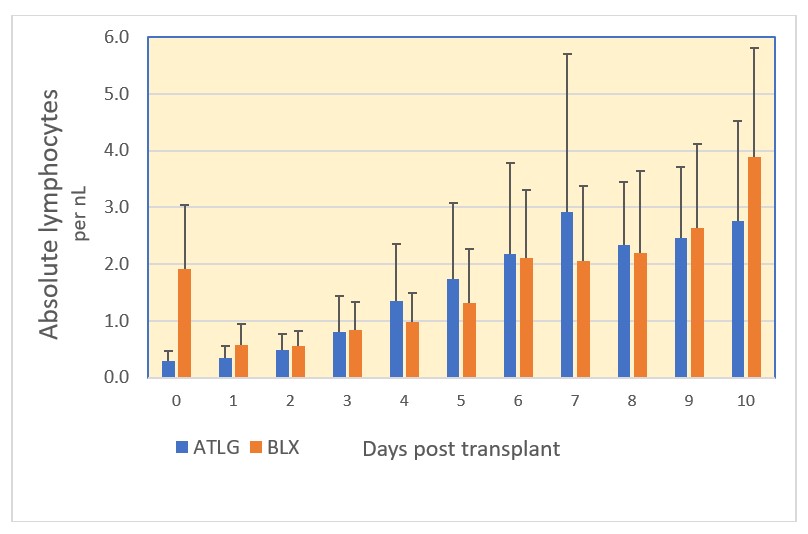
Results Of 44 patients, 38 (86%) received ATLG (26 DD, 12 LRD) and 6 (14%) BLX (5 DD, 1 LRD). 82 % of ATLG patients had 3, the remainder 5 doses (5.1±1.2 mg/kg). Both Abs were tolerated without infusion reactions. Pneumonia was diagnosed in 1 and UTI in 8 children post ATLG. 4/38 ATLG patients developed fever at POD 0-2 without identified cause. There was 1 biopsy-proven BKVN, no symptomatic CMV or EBV infection, no PTLD. ALCs dropped immediately after the first ATLG dose and started to recover after treatment completion (days 3-5 post KT; Fig 1). Delayed lymphocytopenia after BLX was possibly exacerbated by MP. ATLG led to thrombocytopenia <140x10^9/L in 17/38 patients (45%) during the first week compared to 0/6 after BLX (P 0.067). Neutropenia of <1.5x10^9/L occurred in 13 and 33% of patients, respectively (n.s.). Median eGFR (mL/min/1.73m2) at 1 mo post KT was 78 (range 43-233) vs 63 (41-111) after ATLG and BLX, and 73 (35-118; ATLG) vs 64 (42-102; BLX) at one year, respectively. Ten patients developed BPAR corresponding to 0.11 rejections per patient year (RPPY) after ATLG and 0.17 RPPY after BLX. Six rejections occurred during the first year post KT (1 mo, range 0.13-4.93), 4/36 after ATLG and 2/6 after BLX (n.s.).
Conclusion ATLG and BLX were tolerated without serious A/E or opportunistic viral infections. No lasting lymphocyte depletion was noted. Few instances of fever onset during IIS may have been ATLG-related. Rejection rates and graft function were comparable to international standards without significant differences between the induction agents.
Dr Eva Simkova, Dr Entesar Alhammadi, Dr Loai Eid for patient care and stimulating discussions. Dr Leal Herlitz for graft biopsy readings and stimulating discussions.
[1] Immunosuppression
[2] Graft rejection
[3] Anti-T lymphocyte globulin
[4] Basiliximab
[5] Lymphocytopenia
[6] Thrombocytopenia
[7] Pediatrics
[8] Kidney transplantation
