High-dose intravenous immunoglobulin for BK virus DNAemia protects against BK nephropathy but not acute rejection
Hersharan Kaur Sran1, Matthew D'Costa1, Emmett TY Wong1, Zi Yun Chang1, Vathsala Anantharaman1.
1National University Centre for Organ Transplantation, National University of Singapore, Singapore, Singapore
Introduction: BK virus DNAemia (BKV-D) continues to be a burden for kidney transplant recipients (KTRs) with the lack of approved direct antiviral therapies, and the mainstay of treatment being reduction of immunosuppression, which is associated with a risk of acute rejection(AR). Herein, we describe our experience of treatment of BKV-D with high-dose IVIG (HDIVIG) in a multi-ethnic cohort of KTRs.
Methods: In this single-centre, retrospective cohort study we analysed 146 KTRs transplanted between 1-Apr-2019 and 31-Dec-2023. BK DNA levels were screened at monthly intervals post-transplant from months 1-9, then 3-monthly until 24-months post-transplant. Management of BKV-D consisted of halving of anti-metabolite dose followed by cessation, and institution of HDIVIG at 2g/kg every 4-6 weeks for BKV-D > 3log, followed by conversion to mTOR-inhibitors(mTORi), cessation of calcineurin inhibitor(CNI) or other adjunctive therapy. Allograft biopsy was performed in cases of allograft dysfunction. Outcomes of KTRs with BKV-D were manually abstracted from case records.
Results: Baseline characteristics are as per the table below.
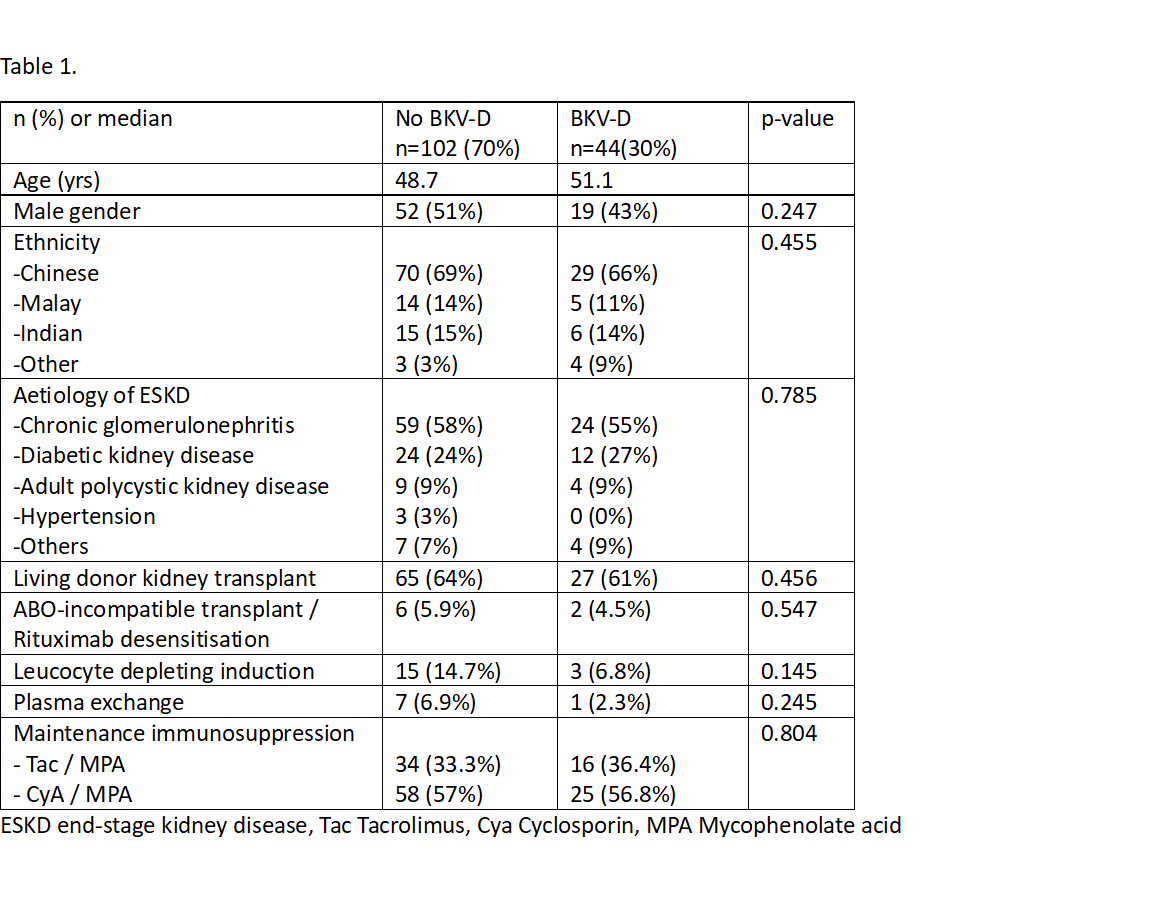
There were no significant differences in demographics, induction or maintenance immunosuppression in KTRs who developed BKV-D vs those who did not. Mean time to detection of BKV-D was 93 days post-transplant (range 26 – 644 days), with time to peak BKV-D noted at 125 days post-transplant. Antimetabolite was stopped in 19(43%), mTORi was commenced in 9(20%), 4 cases received leflunomide and 2 cases had cessation of CNI. 25 KTRs (56%) received HDIVIG. KTRs who received HDIVIG had higher peak BKV-D (4.54log vs 2.98log, p<0.05). KTRs with higher peak BKV-D were more likely to have cessation of anti-metabolite and develop AR. HDIVIG did not significantly affect risk of AR(Fig.1)
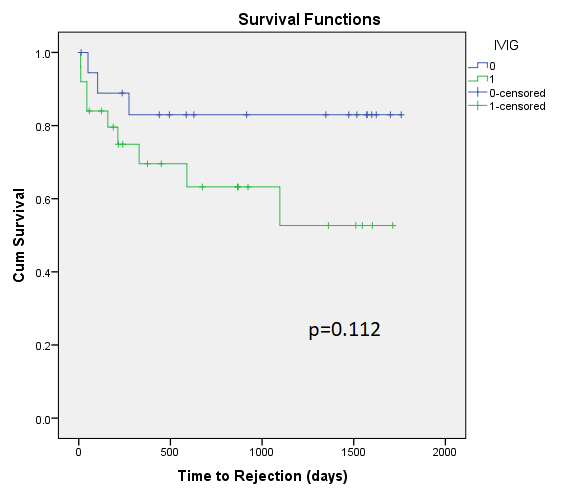
7 KTRs developed AR within 6 months of BKV-D, with 4 preceded by de-novo donor specific antibody development. AR was associated with reduced eGFR at last check (44 vs 59ml/min/1.73m2, p=0.024).
93% of cases resolved, at a median of 68 days (range 16 – 636). Median peak DNAemia was 4.70 log in cases without resolution of BKV-D vs 3.78 log in cases with resolution (p=0.049). There were no cases of biopsy-proven BK nephropathy at median 3.27 years post-transplant, and 1 case of graft loss due to immunosuppression non-compliance.
Conclusions: The high-risk cohort of KTRs with BKV-D >4log requiring aggressive immunosuppression reduction remain at risk of AR and suboptimal allograft function. Further studies are warranted to refine strategies to manage this complex patient cohort.
