Outcomes of liver transplantation for hepatocellular carcinoma in donation after circulatory death compared with donation after brain death
Abdulahad Al-Ameri1.
1Department of Surgery, Zhejiang University , Hangzhou, People's Republic of China
Introduction: Due to organ shortages, liver transplantation (LT) using donationafter-circulatory-death (DCD) grafts has become more common. There is limited and conflicting evidence on LT outcomes using DCD grafts compared to those using donation-after-brain death (DBD) grafts for patients with hepatocellular carcinoma (HCC). We aimed to summarize the current evidence on the outcomes of DCD-LT and DBD-LT in patients with HCC.
Materials and Methods: Online databases were searched for studies comparing DCD-LT and DBD-LT outcomes in patients with HCC and a meta-analysis was conducted using fixed- or random-effects models.
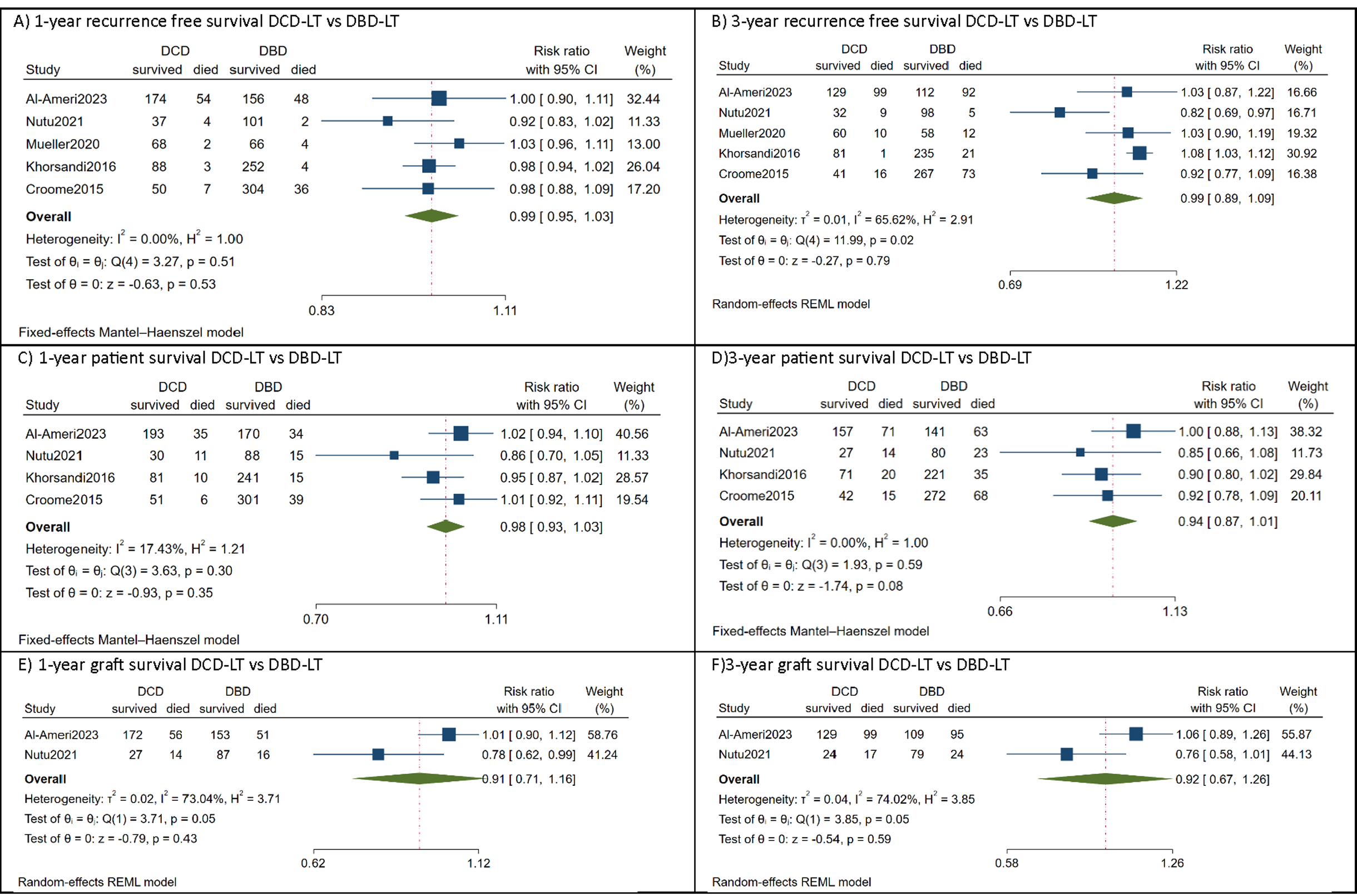
Results: Five studies involving 487 (33.4%) HCC DCD-LT patients and 973 (66.6%) DBD-LT patients were included. The meta-analysis showed comparable 1-year [relative risk (RR)=0.99, 95%CI:0.95 to 1.03, p=0.53] and 3-year [RR=0.99, 95%CI:0.89 to 1.09, p=0.79] recurrence-free survival. The corresponding 1-year [RR=0.98, 95%CI:0.93 to 1.03, p=0.35] and 3-year [RR=0.94, 95%CI:0.87 to 1.01, p=0.08] patient survival and 1-year [RR=0.91, 95%CI:0.71 to 1.16, p=0.43] and 3-year [RR=0.92, 95%CI:0.67 to 1.26, p=0.59] graft survival were also comparable. There were no significant differences between the two cohorts regarding the tumor characteristics, donor/recipient risk factors and the incidence of post-operative complications, including acute rejection, primary non-function, biliary complications and retransplantation.
Conclusions: Based on the current evidence, it has been found that comparable outcomes can be achieved in HCC patients using DCD-LT compared to DBD-LT, particularly when employing good quality graft, strict donor and recipient selection, and effective surgical management. The decision to utilize DCD-LT for HCC patients should be personalized, taking into consideration the risk of post-LT HCC recurrence. (PROSPERO ID: CRD42023445812).